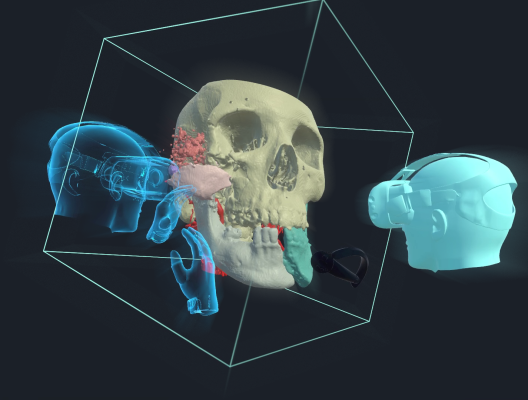
January 20, 2023 — Healthcare software start-up, Realize Medical, announced today that its virtual reality (VR) software for surgical planning, Elucis (ee-loo-sis), has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
“As one of the younRealgest companies in the mixed reality space, FDA clearance is a significant milestone – allowing us to transform our advanced and leading VR software into real benefits for physicians and their patients.”
Elucis enables medical professionals to quickly create, understand, and share complex patient anatomy from CTs and MRIs in an immersive 3D environment. The one-of-a-kind virtual collaboration tool lets users connect with other users in a fully functional virtual reality environment from within VR or through desktop PCs. The platform can be used to improve 3D pre-operative planning and patient consultations.
“Virtual planning with Elucis allows me to conceptualize innovative procedures with the end product of successful cases, development of new devices, and improved education for patients and families,” said Jenny Zablah MD, Congenital Interventional Cardiologist, Colorado.
Current and planned use cases for Elucis are expected to align to the needs of surgeons across all disciplines.
“We’re often told that Elucis represents the most advanced offering in the mixed reality space. But with powerful image processing, modeling, and collaboration tools, it’s also a comprehensive and complete platform for serving the full suite of growing 3D needs in medicine. With FDA clearance, we’re truly excited to enable an expanding era of procedure planning and collaboration.” – Justin Sutherland, CEO
For more information: www.realizemed.com


 February 02, 2026
February 02, 2026 









