March 31, 2009 - Data presented at the ACC in Orlando, FL show that the Rheos Hypertension System from CVRx can lead to significant reductions in hypertension and improvements in heart structure and function.
The Rheos System activates the carotid baroreflex, the body’s own system for regulating blood pressure, and may provide a future treatment option for the millions of people who cannot control their high blood pressure with medications.
“We continue to see favorable clinical results that build on earlier findings from the Rheos clinical studies,” said Marcos Rothstein, M.D., professor of medicine, Division of Renal Diseases, Washington University School of Medicine. “We are optimistic that this novel therapy could offer a new treatment option to patients with drug-resistant high blood pressure. I am impressed with the impact this therapy has had on my patients. Many of them are now able to participate in daily activities that they could not do with uncontrolled blood pressure.”
Dr. Rothstein presented two-year and three-year data from European and U.S. early clinical studies evaluating the safety and clinical effectiveness of the Rheos System as part of the Late-Breaking Clinical Trials III: Emerging Technologies Session (Session No. 407) at the ACC. The findings show a significant reduction in blood pressure in patients with drug-resistant hypertension who have a systolic blood pressure of 160 mmHg or greater, despite being on at least three anti-hypertension medications, including a diuretic. The trials are assessing the safety and clinical efficacy of the Rheos System.
The presentation at ACC reported on office cuff measurement results after two and three years of active Rheos Therapy for the first European and U.S. patients enrolled in these trials at 11 medical centers. After three years of active Rheos treatment, systolic blood pressure was reduced by an average of 31 mmHg in 22 patients. The Rheos implants were well tolerated.
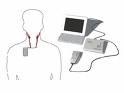
The Rheos System includes:
- A small device that is implanted under the collar bone
- Two thin lead wires that are implanted at the left and right carotid arteries and connected to the pulse generator; and
- The Rheos Programmer System, an external device used by doctors to noninvasively regulate the activation energy from the generator to the lead wires.
The CVRx Rheos System is an investigational device and is limited by Federal (or United States) law to investigational use only.
For more information: www.cvrx.com.


 January 05, 2026
January 05, 2026 









