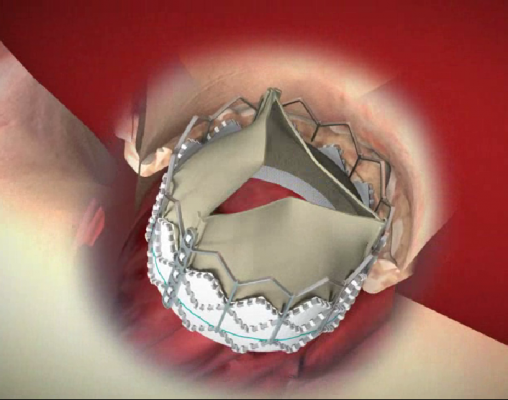
May 23, 2012 - Edwards Lifesciences Corp. announced that data from its post-approval study involving 94 European centers performing transcatheter aortic valve replacement (TAVR) demonstrated positive outcomes for high-risk patients treated with the Edwards Sapien XT transcatheter heart valve. These data document the outcomes of 2,706 consecutively enrolled patients, representing more than 20 percent of the total patients treated with commercially available Sapien XT valves during the time period of this study. These data were presented today at EuroPCR 2012.
Thirty-day outcomes for patients enrolled in the SOURCE XT Registry, a monitored and adjudicated prospective registry studying the Edwards Sapien XT valve in a real-world commercial setting, demonstrated low all-cause mortality of 6.3 percent and low rates of procedural complications. All-cause mortality for transfemoral patients (n=1,694) was 4.3 percent and 9.7 percent for patients treated with all other access routes (n=1,012).
"We are pleased that Heart Teams in Europe continue to achieve positive outcomes with this important therapy in high-risk patients," said Larry L. Wood, Edwards' corporate vice president, transcatheter valve replacement. "Data from this rigorous post-approval study demonstrate the disciplined approach to patient selection that clinicians have maintained in a real-world setting."
The SOURCE XT Registry enrolled patients treated with the Sapien XT valve in 17 countries between July 2010 and October 2011. Patients were treated using a transfemoral (n=1,694), transapical (n=906), transaortic (n=98) or subclavian (n=8) approach and will be followed out to five years.
Edwards also announced at EuroPCR the receipt of CE mark for European commercial sales of its 29 mm Sapien XT valve delivered with the NovaFlex+ transfemoral delivery system. With this valve, Edwards' transcatheter valve portfolio now offers three valve sizes for both transfemoral and transapical delivery, enabling Heart Teams to treat a broader population of transcatheter valve patients better served by a larger valve.
Results from the early first-in-human experiences with Edwards' two new transcatheter valve platforms will be presented during EuroPCR's "Glimpse into the future" session, which is focused on emerging technologies for transcatheter aortic valve therapies. The Sapien 3 valve is Edwards' next-generation balloon-expandable valve that builds upon the company's knowledge and experience in TAVR and includes a unique feature designed to reduce paravalvular leaks. Centera, Edwards' repositionable, self-expanding valve, features a motorized delivery system designed for stable deployment and single operator use. Both systems are low profile and able to be delivered through a 14-French eSheath, Edwards' expandable introducer sheath.
The Edwards Sapien XT valve and the NovaFlex+ transfemoral delivery system are investigational devices not yet available commercially in the United States. The Sapien 3 valve and Centera are investigational devices not yet available commercially in any country.
For more information: www.edwards.com


 January 05, 2026
January 05, 2026 









