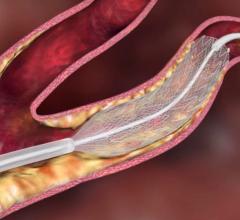
April 10, 2008 - According to a study published in The New England Journal of Medicine this week, three-year data from the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) study in patients with severe blocked carotid arteries, the main blood vessels in the neck leading to the brain, who underwent carotid artery stenting (CAS) with the PRECISE Nitinol Stent and the ANGIOGUARD Emboli Capture Guidewire were comparably protected from stroke, heart attack, death and repeat revascularization procedures as patients who underwent surgery (endarterectomy).
SAPPHIRE is a multicenter, prospective, randomized study that evaluated the safety and performance of the PRECISE Nitinol Stent and ANGIOGUARD Emboli Capture Guidewire, compared with endarterectomy, in 334 patients with high-grade carotid artery stenosis. The prespecified major secondary endpoint at three years was a composite of death, stroke or myocardial infarction within 30 days after the procedure or death or ipsilateral stroke (occurring on the same side) between 31 days and three years.
At three years, data were available for 260 patients, 85.6 percent of patients (143 of 167) in the stenting group and 70.1 percent of patients (117 of 167) in the surgery group. The pre-specified major secondary endpoint occurred in 41 of the 167 patients who underwent stenting (cumulative incidence, 24.6 percent) and in 45 of 167 patients who underwent endarterectomy (cumulative incidence, 26.9 percent). There was no statistical significance between these groups. A procedure to re-open the blocked arteries (target-vessel revascularization) was infrequent in both groups.
"It is important to know the protection of carotid revascularization against future stroke and other ischemic events was maintained at three years and there was no significant difference in the cumulative incidence of major cardiovascular events between carotid revascularization and carotid surgery,"? said Donald E. Cutlip, M.D., executive director of Clinical Investigation, Harvard Clinical Research Institute, Harvard Medical School. Dr. Cutlip currently serves as an investigator in the Cordis-sponsored SAPPHIRE study.
These long-term study results could be significant to the approximately one-third of patients who have severe carotid artery stenosis and require a less invasive, but effective treatment option because they are poor candidates for surgery.
For more information: www.americanheart.org and www.cordis.com

 June 26, 2023
June 26, 2023