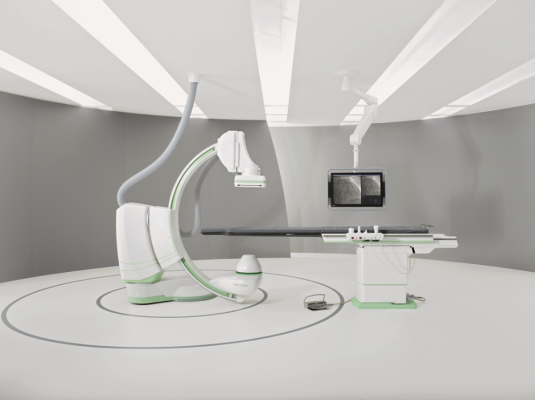
The Siemens Artis one angiography system
October 1, 2014 — St. Johns Hospital and Medical Center in Detroit recently became the first U.S. healthcare facility to install the Artis one angiography system from Siemens Healthcare. The Artis one is designed to address a broad range of angiographic procedures, from diagnosing and treating coronary artery disease and peripheral vessel occlusions to performing electrophysiology procedures. St. Johns is using the Artis one primarily for interventional cardiology and electrophysiology.
“The compact nature of the Artis one angiography system makes it easy to work with,” said Lingareddy Devireddy, M.D., FACC, CCDS, medical director of cardiology services and chief of cardiology at St. Johns Providence Health System in Warren, Mich. “The footprint of the base and its movement make it easy for left-sided implants, and the system’s peristepping function is convenient for peripheral work.”
Offering a positioning flexibility similar to ceiling-mounted systems, the Artis one occupies 269 square feet, compared to the standard 484 square feet required by ceiling-mounted systems. The Artis one has multiple axes capable of moving independently of one another, enabling easy positioning of the system where needed, regardless of where the user is standing. The system accommodates head-to-toe coverage of patients up to 6 feet, 10 inches without the need for patient repositioning.
Artis one’s Clearstent Live application for interventional cardiology procedures — previously available only with Artis Q and Artis Q.zen — freezes motion in the stent region, allowing the physician to mask out movement of the beating heart and place the stent in precisely the correct position. Cardiac imaging tools of the Artis one include HeartSweep, which uses dual-axis rotational angiography to image the entire heart in one smooth C-arm movement to potentially speed up procedures and save contrast agents. By utilizing 20 percent less energy than the Artis zee floor system, the Artis one also helps reduce operational costs.
For more information: www.usa.siemens.com/artis-one


 January 29, 2026
January 29, 2026 









