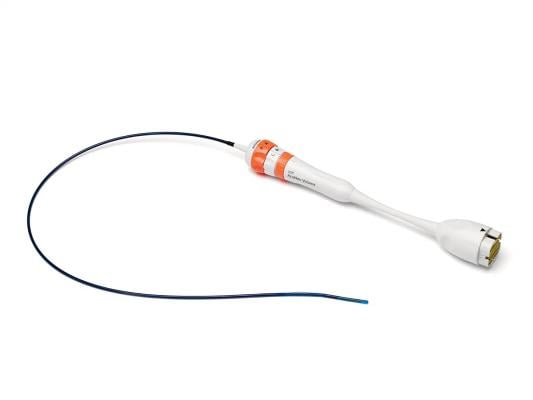
AcuNav Volume ICE catheter
April 12, 2022 – Siemens Healthineers, the global leader in intracardiac echocardiography (ICE), announced the market introduction of the next-generation AcuNav Volume ICE catheter in the United States. With over 10 years of experience in 4D Volume ICE, this new product continues the company’s innovation journey in structural heart and electrophysiology imaging. The AcuNav Volume ICE catheter transforms care delivery by enabling treatment of patients who were previously not able to undergo Structural Heart procedures. This includes patients with TEE (transesophageal echo) or general anesthesia contraindications, as well as patients suffering from tricuspid regurgitation. Additionally, the AcuNav Volume ICE catheter potentially improves hospital efficiency due to reduced cath lab turnaround time and shorter duration of hospital stay, while also eliminating the need of general anesthesia.
The new release of the AcuNav Volume ICE catheter addresses the customer need for advanced precision in Structural Heart procedures. Shortened articulation length of the distal tip is a major update that brings physicians closer to critical anatomy while enhancing maneuverability and control of the catheter during structural heart procedures. This innovative feature supports interventionalists and electrophysiologists in getting greater visualization outcomes and gives them more command over their procedures1.
Since the first-ever human 4D Volume ICE case 10 years ago with the AcuNav V ICE catheter, physician partnership has been core to the company’s continued innovation strategy. Dr. Carlos Sanchez, a long-time Siemens Healthineers partner and Interventional Cardiologist of Advanced Structural Heart Disease, OhioHealth Riverside Methodist Hospital, commented on his first successful hands-on experience with the next-generation AcuNav Volume ICE catheter: “Shorter articulation length of the distal tip enables me to get closer to the anatomy of interest, especially for left heart procedures. This means better imaging outcomes and better care for my patients.”
“Our continued focus is on enabling physicians to improve patient care through innovative imaging products. As structural heart procedures grow in number and complexity, physicians will benefit from having a strong partner with over 10 years of 4D Volume ICE case experience that only we can offer,” said David Zollinger, Head of Intracardiac Imaging, Siemens Healthineers.
The new release of the AcuNav Volume ICE catheter is now commercially available in the U.S.A. and offered exclusively on the ACUSON SC2000 PRIME ultrasound system, a complete Structural Heart Disease solution delivering advanced visualization and AI-powered quantification with 2D and 4D TTE, TEE, ICE and TrueFusion2.
Reference:
1. When compared to the legacy version of the AcuNav Volume ICE catheter.
2. TrueFusion represents a workflow consisting of syngo® TrueFusion (syngo X Workplace) and TrueFusion echo-fluoro guidance (ACUSON SC2000 PRIME).


 October 28, 2025
October 28, 2025 









