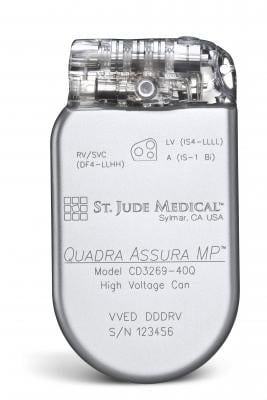
December 14, 2015 — St. Jude Medical Inc. announced CE Mark approval for magnetic resonance (MR) conditional labeling for the company’s currently approved Quadra Assura Cardiac Resynchronization Therapy Defibrillator (CRT-D). The Quadra Assura CRT-D and Quadra Assura MP CRT-D with MultiPoint Pacing are now approved for use with MRI scanning systems with strength up to 1.5 Tesla.
“The Quadra Assura CRT-D MRI allows patients to continue to be actively paced and continuously monitored with the quadripolar pacing technology while having the capability to get an MRI scan if needed,” said Giovanni Forleo, M.D., electrophysiologist at the University Hospital of Tor Vergata, Rome, Italy. “Being able to offer MRI-compatible technology without compromising care decisions is extremely important; this is yet another valuable tool for us to treat patients living with heart failure.”
CRT resynchronizes the beating of the heart's lower chambers (ventricles), which often beat out of sync in heart failure (HF) patients. Studies have shown that CRT can improve the quality of life for many patients with HF, a progressive condition in which the heart weakens and loses its ability to pump an adequate supply of blood. Approximately 23 million people worldwide are afflicted with congestive HF, and 2 million new cases are diagnosed each year worldwide.
The Quadra Assura CRT-D has been shown to demonstrate an improvement in quality of life and reduce hospitalization rates by 53 percent. The Quadra Assura MP CRT-D builds upon that improvement with the MultiPoint Pacing technology on a single left ventricular lead, demonstrated to improve patient response to therapy. HF patients implanted with these devices now have the reassurance of being able to receive an MRI if needed.
The Quadra Assura CRT-D MRI offers the CRT-D device with what the company calls first-to market quadripolar and next-generation Multipoint Pacing technologies, which enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to meet patient needs by optimizing CRT pacing to reduce the rate of CRT non-responders, as well as the likelihood of costly and invasive lead revision through a second interventional procedure.
MultiPoint Pacing is an investigational device and is not commercially available in the United States. MRI labeling is not approved in the U.S. or Australia for these devices.
For more information: www.sjm.com


 July 21, 2025
July 21, 2025 









