In its third quarter financial report, San Diego-based Cytori said it has moved significantly closer to initiating its cardiovascular clinical trials," said Christopher J. Calhoun, chief executive officer of Cytori Therapeutics. The company is developing adipose stem cells in cardiovascular disease and for use in reconstructive surgery.
"The first trial is expected to start before year end in patients suffering from chronic ischemia, a severe form of coronary artery disease, and the heart attack trial is expected to begin in early 2007.
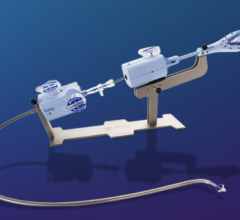
Cytori received U.S. 510(K) regulatory clearance recently on the Celution Cell Concentration System as a cell saver device by the FDA's Center for Devices and Radiological Health (CDRH)
For more information visit www.cytoritx.com


 July 08, 2024
July 08, 2024