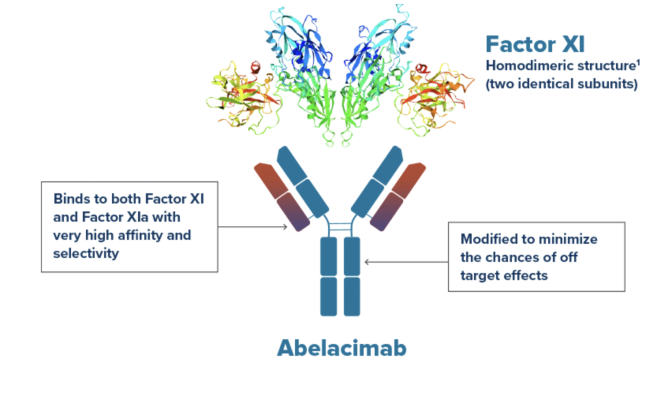
September 26, 2023 — Anthos Therapeutics, Inc., a clinical stage company developing innovative therapies for cardiovascular diseases, founded by Blackstone Life Sciences, announced today that primary data from the AZALEA-TIMI 71study of patients with atrial fibrillation at moderate-to-high risk of stroke has been selected for a Late Breaking session at the American Heart Association Scientific Sessions 2023, being held November 11-13 in Philadelphia.
This news comes just days after it was announced that the AZALEA-TIMI 71 study was stopped early by the independent Data Monitoring Committee (DMC) due to an overwhelming greater-than-anticipated reduction in major and clinically relevant non-major bleeds in abelacimab compared to rivaroxaban and a benefit/risk profile also favoring abelacimab.
“The substantial reduction in bleeding with the monthly administered, dual-acting Factor XI/XIa inhibitor abelacimab compared to a standard-of-care anticoagulant represents an enormous potential advance in the care of patients,” said Principal Investigator Christian T. Ruff, MD, MPH, Director, General Cardiology, Brigham and Women’s Hospital; Senior Investigator, TIMI Study Group; Associate Member, Broad Institute of MIT and Harvard; Associate Professor of Medicine, Harvard Medical School. "The ability of abelacimab to prevent thrombosis with an enhanced safety and tolerability profile will likely not only improve adherence, but also provide physicians with the confidence to extend anticoagulation to the most vulnerable patients who are frequently undertreated or not treated at all."
Late-Breaker Presentation Details
Oral Presentation: Abelacimab, a Novel Factor XI/XIa Inhibitor, vs Rivaroxaban in Patients with Atrial Fibrillation: Primary Results of the AZALEA-TIMI 71 Randomized Trial
- Session Title: LBS.05 – Late Breaking Science: Shocking Decisions in AFib Care
- Date: Sunday, Nov. 12, 2023
- Session Time: 8:00am – 9:15am ET
- Presentation Time: 8:15am – 8:25am ET
- Location: Pennsylvania Convention Center; Philadelphia, Pennsylvania
Anthos Therapeutics and the TIMI Group have initiated an extension study to enable all patients from the rivaroxaban and abelacimab arms to transition to open label abelacimab. A Fast-Track Designation for abelacimab was previously granted by the U.S. Food and Drug Administration (FDA) for the prevention of stroke and systemic embolism in patients with atrial fibrillation.
“This additional positive news further recognizes the confidence that Anthos Therapeutics placed early-on in the development program of abelacimab. The results of the AZALEA-TIMI 71 study firmly establishes that thrombosis can successfully be uncoupled from hemostasis,” said John Glasspool, CEO, Anthos Therapeutics. “We are now turning our full attention, along with our partners at the TIMI Study Group, to advancing our Phase 3 LILAC-TIMI 76 study in patients with atrial fibrillation deemed unsuitable for current anticoagulants, which is estimated to range from 40% to 60% of diagnosed patients.”
For more information: https://anthostherapeutics.com/
Related content:
Gender Differences and AFib: New Study Flips Conventional Wisdom
Global AFib Study Finds Simple Approach is Best for Ablation Procedures


 December 19, 2025
December 19, 2025 









