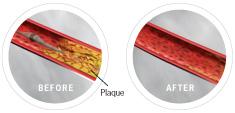
The Diamondback 360 atherectomy catheter approaching a lesion, and following treatment.
April 28. 2010 – A trial examining the use of an orbital atherectomy device on calcified coronary lesions was granted U.S. Food and Drug Administration (FDA) unconditional approval this week.
The ORBIT II pivotal trial will evaluate the safety and effectiveness of the Cardiovascular Systems Inc. (CSI) Diamondback 360 atherectomy system in coronary vessels. CSI received conditional IDE approval for the ORBIT II study in January. The pivotal trial will initially enroll up to 100 patients at as many as 50 U.S. sites, with the potential to enroll up to 429 patients. Jeffrey Chambers, M.D., an interventional cardiologist with Metropolitan Cardiovascular Consultants, Minneapolis, is the principal investigator.
The Diamondback 360° is designed for removing calcific and fibrocalcific plaque. It uses a diamond-coated crown with an orbital mechanism of action to sand and remove hardened plaque, which may facilitate more effective stent placement and restoration of blood flow in the coronary arteries. The orbital action also allows continuous saline and blood flow through the lesion, which may be advantageous during treatment.
In 2008, CSI completed the ORBIT I coronary trial, the first in-human feasibility study, which enrolled 50 patients in India. The Diamondback 360 was shown to be successful in 98 percent of patients with calcified lesions, and the acute procedural success rate, including stent placement, was 94 percent. These results met the company’s safety and efficacy endpoints and were among the data the FDA considered in granting the ORBIT II investigational device exemption (IDE) approval.
“Coronary arterial disease is the most common form of heart disease in the United States, affecting more than 16 million people,” Chambers said. “After the successes of the ORBIT I study, we are eager to expand the number of coronary patients treated with the Diamondback 360°, and acquire acute and long-term data. We believe the unique features of the Diamondback 360° are designed specifically to remove calcified plaque in coronary arteries to facilitate stent placement.”
In August 2007, the FDA granted 510(k) clearance for the use of the Diamondback 360 as a therapy for PAD (peripheral arterial disease), and CSI commenced a U.S. product launch in September 2007.
For more information: www.csi360.com


 November 08, 2024
November 08, 2024 









