March 8, 2010 – Cardiovascular death is the most common cause of mortality among type 2 diabetics and claims the lives of millions each year, with many diabetics experiencing their first “symptom” as a heart attack or sudden death. Understanding that diabetics are at high risk for coronary heart disease (CHD) and many are asymptomatic, Toshiba America Medical Systems Inc. is sponsoring the Speckle Tracking by Echo, a faCTor64 substudy using Toshiba’s wall motion tracking ultrasound technology.
faCTor64 is a landmark study with Intermountain Healthcare to assess asymptomatic diabetics using cardiac CT angiography (CTA) with the Toshiba Aquilion 64 CT multidetector row system.
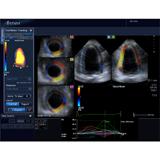
The Speckle Tracking by Echo substudy uses Toshiba’s wall motion tracking technology to evaluate an asymptomatic diabetic population for CHD by detecting if patients have a reduction in myocardial strain. Strain is the percentage change in muscle as the heart contracts. Any reduction in strain indicates a strong possibility of CHD, so the patient is sent to CT for further evaluation. Using Echo to evaluate patients may help physicians detect disease at its earliest stages, before symptoms are present, using noninvasive ultrasound and could avoid patients undergoing invasive, costly procedures such as intervention in the cath lab.
Wall motion tracking works by evaluating one piece or region of the heart muscle and showing how and where that region is moving in relation to other regions of the heart muscle. It is a sensitive test that can show abnormalities and even the slightest change in how a part of the muscle moves, which can be valuable information, but difficult to assess using other methods.
J. Brent Muhlestein, M.D., FACC, director of cardiovascular research, Intermountain Medical Center, and professor of medicine, University of Utah, is leading the Speckle Tracking by Echo ultrasound substudy and the faCTor64 study. He found that many of the asymptomatic diabetic patients have some form of CHD.
“Wall Motion Tracking is a new technique to help us more carefully analyze heart function, specifically the speed of muscle contraction and relaxation of multiple regions of the heart,” explained Dr. Muhlestein. “The goal of this substudy is to determine if the information gleaned through wall motion tracking can be used as an early predictor of adverse cardiac events. So far, wall motion tracking shows significant promise as an inexpensive, noninvasive tool to detect subtle differences in how regions of the heart muscle are working.”
Intermountain Healthcare hopes to enroll more than 300 patients from its diabetes registry, one of the largest in the country, in the Speckle Tracking by Echo substudy. Like faCTor64, the substudy is an outcomes-based trial where, after monitoring both patient groups, researchers will determine which group experienced better outcomes.
As an addition to the substudy, Dr. Muhlestein’s group is also assessing carotid intima-media thickness (carotid IMT) to detect plaque in the carotid artery. Since studies have shown that there is a correlation between carotid plaque and coronary heart disease, Intermountain Healthcare is also using carotid IMT to detect plaque in the carotid as an early predictor.
All clinical studies for the Speckle Tracking by Echo ultrasound substudy and carotid IMT are being performed by Intermountain Healthcare using Toshiba cardiac ultrasound systems. The results are being analyzed using Toshiba wall motion tracking software at Johns Hopkins University School of Medicine, under the direction of Dr. João A.C. Lima, professor of medicine, radiology and epidemiology.
Details About faCTor64
Intermountain Healthcare is using its diabetic database to identify 1,100 diabetic patients, women older than 55 and men older than 50, to enroll in faCTor64. Of this asymptomatic patient population, half will be scanned to detect if CHD is present and if intervention is needed, while the other half will not be scanned and will only undergo traditional diabetic management. After five years of monitoring, researchers will determine which group experienced better outcomes.
For more information: www.medical.toshiba.com


 January 05, 2026
January 05, 2026 









