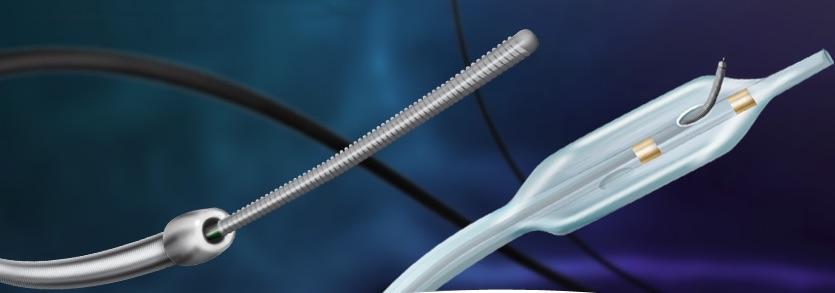
November 2, 2017 — Crossing the occlusion with a guidewire is often the most challenging part of chronic total occlusion (CTO) percutaneous coronary intervention (PCI), and there is debate about the optimal initial crossing strategy during the procedure. Researchers randomized 246 patients undergoing coronary CTO intervention to upfront use of the CrossBoss catheter (N=122) or wire escalation (N=124) at 11 U.S. centers. Overall technical and procedural success rates were high and similar between the two groups. The primary efficacy endpoint, crossing time, was similar: 56 (interquartile range: 33, 93) minutes in the CrossBoss and 66 (36, 105) minutes in the wire escalation group (P=0.323), as was the incidence of procedural major adverse cardiovascular events (3.28 percent vs. 4.03 percent, P= 1.000). In the CrossBoss-first strategy, the first crossing technique was more likely to be antegrade dissection/re-entry (77 percent), compared with the wire escalation strategy, which was successful as the initial strategy 98 percent of the time among patients randomized to that study arm.
Trial results were presented at the Transcatheter Cardiovascular Therapeutics (TCT) 2017 annual conference, Oct. 29-Nov. 2 in Denver.
“The CrossBoss First trial is the first randomized-controlled trial designed to compare two commonly utilized crossing techniques as the initial CTO crossing strategy,” said Emmanouil S. Brilakis, M.D., of the Minneapolis Heart Institute. “Compared with a primary wire escalation strategy, upfront use of the CrossBoss catheter for crossing CTOs was associated with similar crossing time, similar success and complication rates, and similar equipment utilization and cost. In a post hoc analysis upfront use of the CrossBoss catheter was associated with shorter crossing time in patient with in-stent CTOs. Use of antegrade dissection/re-entry was the final successful strategy in 22 percent of the wire escalation group patients.”
The CrossBoss First study was funded by Boston Scientific. Brilakis reported consulting/speaker honoraria from Abbott Vascular, Amgen, Asahi, CSI, Elsevier, GE Healthcare and Medicure. He also disclosed research support from Boston Scientific and Osprey, and that his spouse is an employee of Medtronic.
Read about other TCT 2017 late-breaking clinical trial presentations.
For more information: www.tctconference.com
Related CTO Content
VIDEO: Treating Chronic Total Occlusions
VIDEO: New Technology to Treat Chronic Total Occlusions (CTOs)
When to Consider Revascularization of Coronary Chronic Total Occlusions


 November 14, 2025
November 14, 2025 









