February 12, 2009 - Researchers in The Netherlands say the standard of care of using intra-aortic balloon pumps (IABP) in patients with high-risk STEMI does not appear to help patients, according to a meta-analysis conducted by the Department of Cardiology, Academic Medical Center, University of Amsterdam.
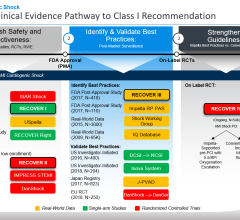
The study was recently posted on the Web site for the European Heart Journal. Intra-aortic balloon counterpulsation in ST-segment elevation myocardial infarction (STEMI) with cardiogenic shock is strongly recommended in the current guidelines from several cardiac organizations (ACC, ESC and AHA). Dr. José P.S. Henriques, one of the study authors, said the meta-analyses was performed to evaluate the evidence for IABP in STEMI with and without cardiogenic shock. They searched medical literature databases to identify randomized trials comparing IABP with no IABP in STEMI. In absence of randomized trials, cohort studies of IABP in STEMI with cardiogenic shock were identified.
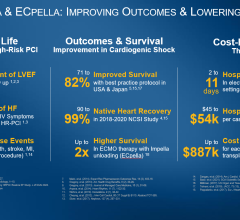
Two separate meta-analyses were performed respectively. The first meta-analysis included seven randomized trials (with a total of 1,009 patients) of STEMI. The study showed IABP showed neither a 30-day survival benefit nor improved left ventricular ejection fraction, while being associated with significantly higher stroke and bleeding rates. The second meta-analysis included nine cohorts of STEMI patients with cardiogenic shock (n = 10,529). In patients treated with thrombolysis, IABP was associated with an 18 percent decrease in 30-day mortality, albeit with significantly higher revascularization rates compared to patients without support. In patients treated with primary percutaneous coronary intervention, IABP was associated with a 6 percent increase in 30-day mortality, Dr. Henriques said.
The study concluded the pooled data does not support IABP in patients with high-risk STEMI and there is insufficient evidence to endorse the current guideline recommendation for the use of IABP therapy in the setting of STEMI complicated by cardiogenic shock.
For more information: http://eurheartj.oxfordjournals.org


 April 28, 2023
April 28, 2023