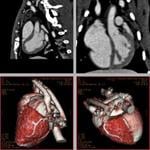
3D rabbit heart spiral CT images enhanced using the N1177 contrast agent.
June 12, 2009 - NanoScan Imaging LLC today announced the publication of new data demonstrating the use of its investigational, radio-opaque contrast agent (N1177) to visualize vulnerable plaques that can cause heart attack or stroke using advanced, noninvasive and high-resolution computed tomography (CT) techniques.
Results of the study were published in the current issue of the Journal of Nuclear Medicine (June 2009, pages 959-965).
N1177, an emulsified suspension that is composed of crystalline iodinated particles dispersed with surfactant, is being developed to visualize blood vessels and organs of the body, with particular interest in the arteries, veins and the heart chambers using a technique known as CT angiography (CTA). N1177 also accumulates in macrophage cells allowing for their detection with CT.
Macrophages are the predominant cells involved in creating the progressive plaque lesions of atherosclerosis, a progressive disease and the main cause of cardiovascular disease - the number one killer worldwide. Atherosclerosis is caused by the build-up of plaques (fatty or fibrous deposits) in the artery walls. This can result in the narrowing of the arteries, which can reduce the supply of blood to vital organs such as the heart and brain. Plaques can also rupture, leading to a sudden, complete blockage of blood flow. Plaques that are at high-risk or “vulnerable” to rupture are characterized by strong macrophage infiltration, which result in acute plaque destabilization and thrombus formation.
In the study, researchers from the Mount Sinai School of Medicine investigated whether or not N1177 correlated with macrophage activity evaluated with 18fluorine-fluorodeoxyglucose (FDG) on PET/CT and also macrophage density on histology. After only two hours, the enhancement of the macrophage rich plaque after the injection of N1177 was significantly higher and specific inside of the vessel wall in rabbits fed a high cholesterol diet compared to control rabbits fed a normal chow diet (p
Several noninvasive imaging techniques have been investigated for macrophage detection in atherosclerotic plaques. For example, ultrasmall superparamagnetic particles of iron oxide (USPIO) are taken up by macrophages that can be detected as signal voids using magnetic resonance imaging (MRI) or combined with a radiotracer to quantify the accumulation of these particles using PET. However, high spatial and temporal imaging resolutions required for imaging the arterial wall of coronary arteries are currently achievable neither with PET nor with MRI. In addition, the optimal imaging time after the injection of iron oxide nanoparticles may be up to 72 hours, limiting the practical use of this technique as a screening tool.
By comparison, CT offers to detect macrophage-rich lesions as early as two hours after the intravenous injection of N1177 with a spatial resolution allowing for the evaluation of coronary atherosclerotic plaques. Quantification of N1177 accumulation in atherosclerotic plaques is facilitated by the linear relationship existing between iodine concentration in tissue and signal increase measured with CT. In addition, detection of macrophages with N1177 could be used in connection with other CT markers identified in ruptured plaques such as areas of low densities, positive remodeling and absence of calcifications to enhance the potential of CT to identify high-risk plaques.
N1177 is cleared through the liver unlike traditional contrast agents that are cleared through the kidneys, which can result in renal toxicity.
For more information: www.nanoscanimaging.com


 January 05, 2026
January 05, 2026 









