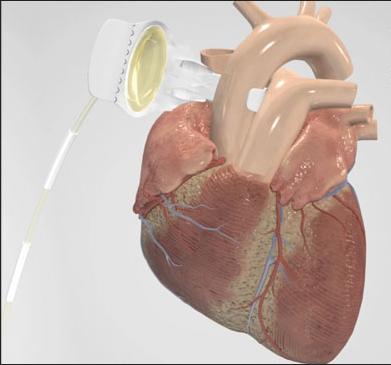
C-Pulse System image courtesy of Sunshine Heart Inc.
May 4, 2015 — Sunshine Heart Inc. announced that the U.S. Food and Drug Administration (FDA) has reviewed the company’s submission regarding the pause of the COUNTER HF U.S. pivotal study. The FDA has requested minor protocol changes be submitted in order to receive approval to resume patient enrollment.
The FDA did not indicate concerns regarding safety of the C-Pulse device and requested the updated protocol include information on several minor items, the most significant of which are the details regarding the company's proposal to incorporate a Physician Subject Selection Committee. Furthermore, the Data Safety Monitoring Board (DSMB) reviewed COUNTER HF's data and recommended continuing the study.
Sunshine Heart previously announced on March 6, 2015, a temporary pause from enrollment in accordance with the study protocol. The protocol indicated that in the event more than three of the first twenty subjects pass away for any reason, including non-device related deaths, the company would work with the FDA to establish a plan before resuming enrollment. All four of the reported patient deaths have been adjudicated by an independent Clinical Events Committee (CEC) as being non-device related.
COUNTER HF is a prospective, randomized, multi-center, controlled study evaluating the safety and efficacy of the C-Pulse system for the treatment of New York Heart Association (NYHA) Class III and ambulatory Class IV heart failure. The study is being conducted by heart failure, electrophysiologists and cardiac surgeon specialists in the United States. It is expected to randomize 388 patients in up to 40 clinical sites. The purpose of the study is to determine whether the C-Pulse System is a safe and effective treatment for heart failure patients who meet the following key study qualifications:NYHA Class III or early Class IV heart failure*;
- Ejection fraction less than or equal to 35% (measure of how well the heart pumps blood);
- Taking appropriate heart failure medications as prescribed by doctor; and
- Have been evaluated for cardiac resynchronization therapy with or without defibrillation (CRT, CRT-D) or implantable cardioverter defibrillator (ICD) therapy.
The C-Pulse Heart Assist System utilizes the scientific principles of intra-aortic balloon counter-pulsation applied in an extra-aortic approach to assist the left ventricle by reducing the workload required to pump blood throughout the body, while increasing blood flow to the coronary arteries. Combined, these potential benefits may help sustain the patient's current condition or, in some cases, reverse the heart failure process, thereby potentially preventing the need for later-stage heart failure devices, such as left ventricular assist devices (LVADs), artificial hearts or transplants. It may also provide relief from the symptoms of Class III and ambulatory Class IV heart failure and improve quality of life and cardiac function. Based on the results from our feasibility study, we also believe that some patients treated with our C-Pulse System may be able to stop using the device due to sustained improvement in their conditions as a result of the therapy.
For more information: www.sunshineheart.com


 January 05, 2026
January 05, 2026 









