August 14, 2012 — Berlin Heart announced that a study recently published in the New England Journal of Medicine (NEJM) concluded that survival using the Excor pediatric ventricular assist device (VAD) is "significantly greater" than the standard-of-care (extracorporeal membrane oxygenation, or ECMO) as a bridging therapy for children in need of a heart transplant. The principal author, Charles D. Fraser, Jr., M.D., surgeon-in-chief and head of the division of congenital heart surgery at Texas Children's Hospital, served as national principal investigator for the clinical trail.
The trial was a prospective, multicenter, single-arm cohort study. Children implanted with the Excor pediatric VAD as a bridge to cardiac transplantation were compared to a historical control group of children supported by ECMO that were selected from the Extracorporeal Life Support Organization registry. Seventeen pediatric cardiac centers in the United States and Canada participated in the trial.
A total of 48 patients in need of support (ages 16 and under) were divided into two cohorts based on body size. Each cohort included 24 patients, Cohort 1 being the smaller participants and Cohort 2 comprised the larger participants. Among participants in Cohort 1, median time of support was 27 days in contrast to the matched ECMO control median support time of five days. In Cohort 2, the median support time was 43 days whereas the ECMO control group was supported a median of five days. Overall, 88 percent of participants in Cohort 1 and 92 percent of participants in Cohort 2 survived to either heart transplantation or weaning with the use of the Excor pediatric VAD.
"The Excor pediatric ventricular assist device represents a major medical advance in the treatment of children who need a heart transplant," said Fraser. "Most of these babies and children would not otherwise survive without the support of Excor while awaiting donor hearts."
"Until the [U.S. Food and Drug Administration] FDA approved Excor, options for mechanical circulatory support as a bridge to heart transplantation in children with severe heart failure were finite," added Robert Kroslowitz, president and CEO of Berlin Heart's North American operations. "More specifically, the effective period of ECMO support is typically limited to only 10 days before life-threatening complications ensue that often preclude transplantation. The narrow duration of support afforded by ECMO is often inadequate considering current waiting times for a pediatric heart transplant (a median of 119 days for all infants in 2008). Consequently, less than 50 percent of the children supported on ECMO survive to undergo heart transplantation."
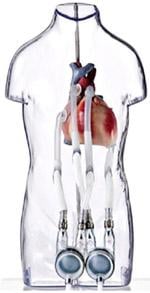
The Berlin Heart Excor pediatric VAD is a mechanical cardiac support system for critically ill pediatric patients suffering from severe heart failure. The system is designed to support pediatric patients of all age groups, from newborns to teenagers, and is intended to bridge patients awaiting heart transplantation from days to many months, until a donor heart becomes available. The FDA granted humanitarian device exemption (HDE) approval in December 2011. The device is also approved for use in Europe and Canada.
For more information: www.berlinheart.com


 January 05, 2026
January 05, 2026 









