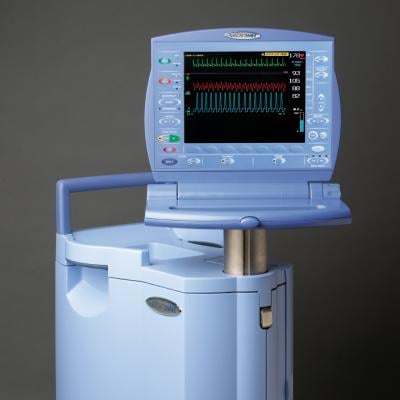
Teleflex Inc. announced a new agreement with HealthTrust Purchasing Group L.P. (HealthTrust), for its Arrow intra-aortic balloon pump and catheter products. The new agreement begins March 1, 2012 and extends through Feb. 28, 2015. HealthTrust is a group purchasing organization supporting nearly 1,400 not-for-profit and for-profit acute care facilities, as well as 10,600 ambulatory surgery centers, physician practices and alternate care sites. Teleflex, through its Arrow Cardiac Care Products, offers intra-aortic balloon (IAB) catheter and intra-aortic balloon pump (IABP) products.
The AutoCAT2 WAVE pump, combined with Arrow’s FiberOptix IAB catheter, offers ProActive CounterPulsation. Through the patented WAVE timing algorithm, converting arterial pressure to aortic flow, combined with the AutoCAT2’s Deflation Timing Management and second generation AutoPilot mode of operation, the intra-aortic balloon pump system enables the clinician to support even the most challenging heart rhythms with 98 percent timing accuracy.
In addition, Teleflex’s Cardiac Care division offers conventional catheters, ranging from polyurethane to wire-reinforced catheter bodies in a variety of balloon membrane sizes to match physician preference and patient need. The AutoCAT2 series also offers a smaller and lighter weight pump configuration for situations where pump weight is important, such as during helicopter transport of critically ill cardiac patients who require IABP support.
“We are proud to be a contracted partner with HealthTrust and look forward to the new opportunities this agreement brings to serve HealthTrust members and their patients,” said Howard Miller, president of cardiac care division.
For more information: www.teleflex.com


 August 14, 2023
August 14, 2023 









