January 24, 2014 —
Peripheral artery disease (PAD) sufferers maintain improved quality of life, including being able to walk farther, three years after being treated with stents to open their blocked leg arteries, according to STROLL
trial results presented at the 26th annual International Symposium on Endovascular Therapy (ISET).
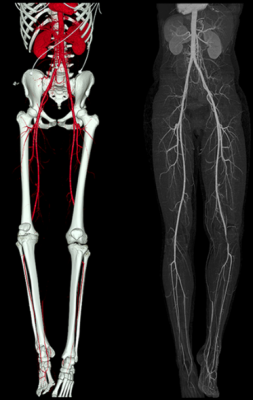
Leg arteries remained open in nearly three-quarters of patients who were treated with nickel titanium (nitinol)
stents, and they continued to enjoy improved quality of life, according to the study.
In the study, self-expanding stents were placed in blockages in the superficial femoral artery of 250 patients. Blockages averaged 7.7 cm, and 23.6 percent of them were completely closed. Three years after treatment, 72.7 percent of arteries remained opened in 209 patients. 3.6 percent of stents had fractured, but all were the least-severe form and caused no problems while continuing to keep arteries open. Blood pressure in the legs remained significantly improved, with almost no change over three years. Further, patients maintained their improved health-related quality of life as measured by several factors, including symptoms and walking distance and speed.
STROLL (S.M.A.R.T. Nitinol Self-Expanding Stent in the TReatment of Obstructive SuperficiaL FemoraL Artery Disease) is a multicenter, nonrandomized, single-arm prospective trial studying the safety and efficacy of the S.M.A.R.T. stent. Patients were treated at one of 39 centers.
Find out more: www.iset.ge