November 11, 2014 — TIDI Products LLC has acquired CFI Medical Solutions (CFI) of Fenton, Mich., a diversified medical device manufacturer and engineering resource for hospitals, distributors and global original equipment manufacturers.
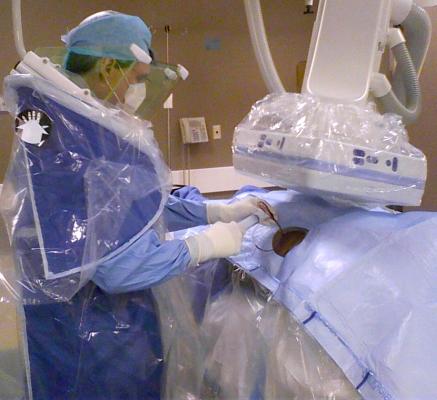
CFI has expertise in the design, engineering and manufacturing of radiation protection, infection prevention and patient positioning systems that help clinicians improve the effectiveness of their practices and healthcare systems. The company’s innovation process is collaborative, developing products by working side-by-side with clinicians and physicians.
“We’ve seen some of the value added innovations, such as C-Armor and Zero-Gravity that CFI has developed in partnership with its healthcare professional network” said Kevin McNamara, president and CEO of TIDI. “We look forward to adopting CFI’s culture of innovative thinking throughout our business to further enhance TIDI’s mission of supporting caregivers and preventing infections.”
C-Armor is an expandable and collapsible sterile pouch that adheres to the patient drape at or above the level of the sterile field line during surgical procedures. Zero-Gravity is a suspended radiation protection system that provides greater protection to clinicians during fluoroscopic procedures.
Headquartered in Neenah, Wis., TIDI plans to continue CFI’s engineering and manufacturing operations for its domestic and international customers. CFI employs approximately 300 people at its manufacturing locations in Fenton, Mich., and its Changshu Yushan Protection Products Co. Ltd. facility in Changshu, China.
For more information: www.tidiproducts.com, www.cfimedical.com


 November 14, 2025
November 14, 2025 









