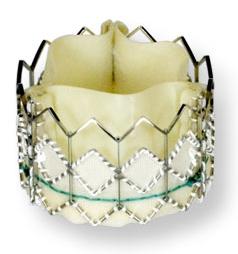
June 4, 2010 – One-year mortality rates were very low for 1,038 high-risk patients, many of whom were too sick to undergo traditional open-heart surgery, who received the Sapien transcatheter heart valve. Results of the SOURCE Registry were presented last week at EuroPCR in Paris.
The registry is the largest reported experience in the world with one-year adjudicated data on consecutive patients treated with transcatheter heart valves. It showed a one-year survival rate of 81.1 percent in transfemoral procedures (valve delivered via the femoral artery) and 72.1 percent in transapical procedures (valve delivered via a small incision through the ribs).
"The encouraging outcomes add to the evolving body of clinical evidence that demonstrates transcatheter aortic valve implantation is a viable option for this high-risk patient population. The data provide valuable, real world insights that enable the continued advancement of this important treatment for patients in need of alternative therapies to traditional open-heart surgery," said Martyn Thomas, M.D., director of cardiothoracic services, Guys and St. Thomas' National Health Service (NHS) Foundation Trust in London.
The SOURCE Registry includes outcomes data from 100 percent of patients treated with the Edwards Sapien transcatheter heart valve at 32 European centers from November 2007 to January 2009 in its initial Cohort I. One year follow-up data was obtained on 98 percent of studied patients. Thomas also announced that 30-day results on an additional cohort of 1,301 patients (Cohort 2) enrolled in 2009 will be reported in the fall.
"The SOURCE Registry is unprecedented in its rigor as a commercial registry, which is critical for our understanding of the developing therapy of transcatheter aortic valve implantation. The one-year outcomes continue to demonstrate that European clinical teams are translating early clinical learnings into successful therapy in an appropriate patient population," said Olaf Wendler, M.D., Ph.D., clinical director for cardiology and cardiothoracic surgery, King's College Hospital in London.
Thomas and Wendler provide paid consulting services to Edwards for education, and research and development of transcatheter valve technologies.
For more information: www.edwards.com


 January 05, 2026
January 05, 2026 









