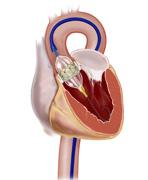
November 16, 2010 – Analysis of data from the PARTNER trial showed that patients implanted with transcatheter heart valve not only experienced improved survival, but also substantially better quality of life. The results, which were presented at the American Heart Association's Scientific Sessions 2010, focused on the Sapien transcatheter heart valve, by Edwards.
"The degree and immediacy of the quality of life improvement was striking, with significant benefits seen as early as one month. By one year, patients experienced both cardiovascular and physical health benefits, with the physical improvements roughly comparable to a 10-year reduction in age," said David J. Cohen, M.D., M.Sc., director of cardiovascular research at St. Luke's Mid-America Heart and Vascular Institute, and principal investigator for the quality of life sub-study that was funded by Edwards. "Quality of life is critically important, particularly for patients like those in this trial - and they are not just surviving, but also thriving."
The patients were assessed upon enrollment and at three follow-up intervals on a broad range of factors, such as their symptoms, physical and social limitations, and heart-failure related quality of life.
On a scale from 0 to 100, where a 20-point improvement is considered substantial, the transcatheter valve patients had a 25-point improvement compared to the control group at one year. Similar positive results were demonstrated for the other two surveys.
The Sapien transcatheter valve is an investigational device in the United States.
For more information: www.edwards.com


 January 05, 2026
January 05, 2026 









