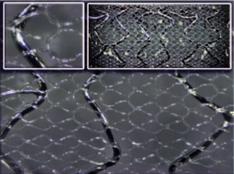
September 28, 2010 – A new multinational trial will examine the effectiveness of embolic protection mesh stents in STEMI (ST-elelvated myocardial infarction) patients.
The MASTER trial will compare the MGuard coronary stent, made by InspireMD, with standard care. The MGuard stent is covered in a polymer mesh to prevent emboli from escaping when the stent is expanded.
“Too often, primary PCI (percutaneous coronary intervention) patients with heart attack results in suboptimal blood flow to the heart muscle, resulting in excessive heart damage,” said Gregg Stone, M.D., study chairman and director of cardiovascular research and education from Columbia University in New York. “We are hopeful that this trial will demonstrate that the MGuard technology improves the success rate of PCI and clinical outcomes in patients with heart attacks.”
The trial will run in Europe, South America and Israel with principal investigators Alexandre Abizaid, M.D., Dariusz Dudek, M.D., and professor Chaim Lotan.
For more information: www.inspire-md.com


 January 05, 2026
January 05, 2026 









