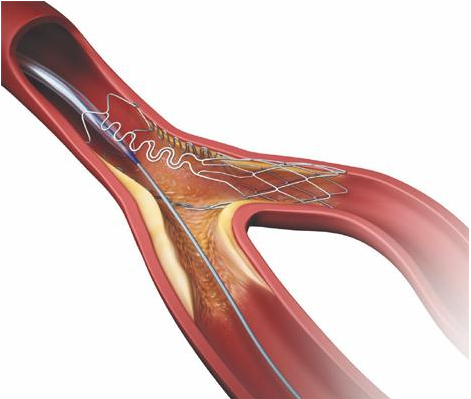
October 28, 2016 – Tryton Medical Inc., a primary developer of stents to treat coronary bifurcation lesions, and Cardinal Health today announced that the companies have established a long-term strategic agreement for U.S. distribution of the Tryton Side Branch Stent, pending regulatory approval. Tryton submitted a pre-market approval (PMA) application to the U.S. Food and Drug Administration (FDA) for the Tryton Side Branch Stent in November 2015.
Bifurcation lesions are present in 20-30 percent of patients with coronary artery disease (CAD) who are treated with percutaneous coronary intervention (PCI). If approved, the Tryton Side Branch Stent would be the first stent specifically indicated for the treatment of bifurcation lesions in the United States.
Read the article "Tryton Completes Patient Enrollment in Pivotal FDA Side Branch Stent Trial."


 November 14, 2025
November 14, 2025 









