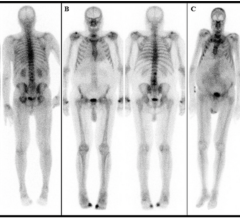
April 21, 2016 – UltraSPECT Inc. announced the 10th anniversary of the U.S. Food and Drug Administration (FDA) approval of its Wide Beam Reconstruction (WBR) technology. The technology employs an iterative reconstruction imaging algorithm to enable lower dose and shorter time imaging for nuclear medicine exams.
An early entrant to the market, UltraSPECT has had double-digit growth in both annual revenue and annual units sold for the last few years. Factors like the recently introduced American Society of Nuclear Cardiology (ASNC) low-dose guidelines, heightened patient awareness to the risks of exposure to radiation, and new Centers for Medicare and Medicaid Services (CMS) reimbursement penalties have accelerated the quest by hospitals to implement emerging low-dose and ultra-low dose nuclear medicine technologies.
In 2006, UltraSPECT introduced to the U.S. market the first application for shorter-time patient imaging both for oncology and cardiology exams, and followed that with the enhanced technology for both shorter-time and lower-dose imaging in 2008. The resulting benefit is enhanced value to physicians, technologists, patients, administrators and radiopharmaceutical suppliers of reduced dose, shorter scans and increased patient comfort with images as good, if not better in diagnostic quality, than those provided with traditional single photon emission computed tomography (SPECT) acquisition.
UltraSPECT has achieved the following key milestones:
- Double-digit revenue growth for the past three years;
- More than 200 units sold between 2013-2015;
- Installations in 46 states;
- 16 distribution partners, including 8 different radiopharmaceutical pharmacies;
- Compatibility with 41 different gamma cameras and 12 workstations representing all the major manufacturers; and
- 100% field validation
“What started as a handful of installations have evolved into tremendous market demand as everyone — from patients and technologists to physicians, pharmacies and pharmacy staff — is benefiting,” said Wayne Wong, owner and vice president, Nuclear Diagnostic Products. “We believed in the product from the beginning and trusted that the nuclear medicine would catch on to the low-dose movement, and being an early partner of UltraSPECT has been a great decision for us.”
While early adopters were instrumental in setting the foundation for UltraSPECT, recent expansion has been in part due to a number of other market conditions, including changes from regulatory groups. They include the ASNC guidelines for low-dose imaging that took effect in January 2015, along with the Physician Quality Reporting System (PQRS) requirement to obtain an additional 2 percent in CMS reimbursement. Other industry pressures influencing the market are:
- Growing shortage of Tc-99m isotope;
- Greater awareness by patients of the need for low-dose imaging supported, in part, by action groups like Image Wisely, launched at the Radiological Society of North America (RSNA) 2010 annual meeting; and
- Growing cost pressures. At a fraction of the cost of a brand-new camera, UltraSPECT also provides one of the most cost-effective solutions to the nuclear medicine market, according to the company.
For more information: www.ultraspect.com


 November 12, 2025
November 12, 2025