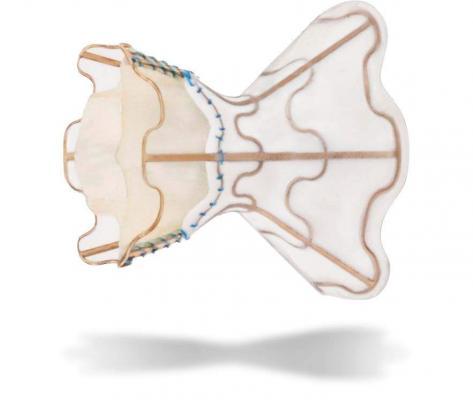
May 16, 2018 — Israel-based V-Wave Ltd. recently closed a $70 million Series C financing for its proprietary, minimally invasive implanted interatrial shunt device for treating patients with severe symptomatic heart failure (HF). The financing round was led by Deerfield Management, along with participation from new investors – healthcare funds Endeavour Vision, Quark Venture and Aperture Venture Partners. All of V-Wave's existing major investors are also participating in this round, including strategic investors Johnson & Johnson Innovation (JJDC Inc.) and Edwards Lifesciences, along with BRM Group, Pontifax, Pura Vida Investments, TriVentures, BioStar Ventures and Israel Secondary Fund.
Having received approval from the U.S. Food and Drug Administration (FDA) to initiate a pivotal investigational device exemption (IDE) study, V-Wave also announced the upcoming launch of its global, randomized, controlled, double-blinded multicenter clinical trial. The RELIEVE-HF study will evaluate the safety and effectiveness of V-Wave’s novel device therapy in HF patients with Class III or ambulatory Class IV symptoms with preserved or reduced ejection fraction already receiving optimal therapies.
With more than 26 million people suffering from chronic heart failure globally, including more than 6 million people in the U.S., heart failure is the leading cause of hospitalizations in many countries. In the U.S., it is Medicare's largest expense for acute hospitalization. Heart failure patients experience repeated hospitalizations, a poor quality of life and a greatly reduced life expectancy.
"V-Wave has developed a novel technology that addresses the underlying cause of heart failure decompensations," remarked Andrew ElBardissi, M.D., principal at Deerfield Management. "This technology has the potential to be the standard of care for a large segment of HF patients that continue to worsen despite the use of approved drugs and devices. In addition to improving outcomes for patients, this has the potential to significantly reduce the cost burden of heart failure."
"V-Wave's interatrial shunt provides clinicians a new tool to control elevated left atrial pressure, the primary cause of breathing difficulty and hospitalization due to worsening HF," noted William T. Abraham, M.D., professor and chief of cardiovascular medicine at the Ohio State University Wexner Medical Center. "The company's feasibility study results, presented in March 2018 as a late-breaking clinical trial at the American College of Cardiology, showed that shunting was safe, and that morbidity and mortality were low compared to a matched population receiving optimal care. The upcoming pivotal trial in at least 400 randomized patients should provide sound assurance of the efficacy of this approach in patients that have a poor prognosis and few options."
For more information: www.vwavemedical.com


 November 14, 2025
November 14, 2025 









