VenaSeal Closure System
Related Content
Sept. 9, 2025 — The American Venous Forum (AVF), International Society on Thrombosis and Haemostasis (ISTH), National ...
October 14, 2022 — Medtronic, a global leader in medical technology, announced the 36-month final results from the ABRE ...
November 22, 2021 — Fewer than 10 days of low-molecular-weight heparin (LMWH) after stenting for extensive iliofemoral ...
February 22, 2021 — The U.S. Food and Drug Administration (FDA) recently cleared the Cook Medical Zilver Vena Venous ...
October 26, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the Medtronic Abre venous self-expanding ...
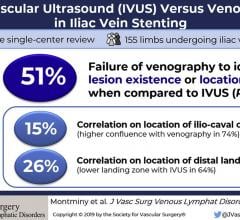
October 31, 2019 – In a large series of iliac vein stent cases, a blinded comparison found intravascular ultrasound ...
May 6, 2019 — The U.S Food and Drug Administration (FDA) has cleared the Boston Scientific Vici Venous Stent System for ...
April 3, 2019 — The U.S. Food and Drug Administration (FDA) recently cleared Bard Peripheral Vascular's Venovo Venous ...
August 8, 2018 — Boston Scientific Corp. announced it has signed an agreement to acquire Veniti Inc., which has ...
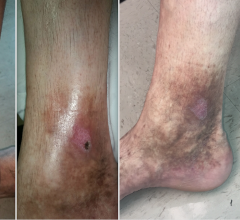
The introduction of thermal ablation revolutionized the treatment of varicose veins, yet recurrence remains a stubborn ...


 September 10, 2025
September 10, 2025