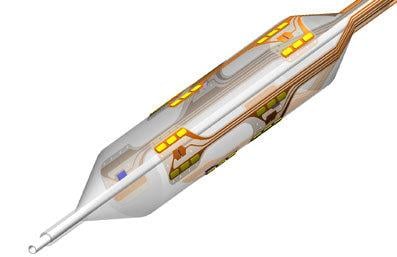
May 2, 2012 — Vessix Vascular Inc., a developer of novel percutaneous radiofrequency (RF) balloon catheter technology for the treatment of hypertension, announced this week that it has received CE mark approval for its V2 Renal Denervation System for the treatment of hypertension. Renal denervation is a percutaneous catheter-based therapy that uses RF energy to disrupt renal sympathetic nerves whose hyperactivity leads to uncontrolled high blood pressure.
The CE mark enables Vessix to market its patented V2 System throughout the European Union. The CE certification was issued to Vessix by its notified body, BSI Group, also known as the British Standards Institution.
"Vessix offers a unique approach to treating uncontrolled hypertension that provides excellent clinical results and is faster, easier to use and less painful for patients than [other] renal denervation systems," said Vessix CEO Raymond W. Cohen. "After eight years optimizing RF balloon catheter and bipolar RF generator technologies, it is rewarding to see the V2 System working effectively in clinical practice to safely reduce patient blood pressures. As part of our commercialization strategy, Vessix has initiated a post-market approval surveillance study during which we plan to treat 120 patients at up to 20 international centers located across Western Europe."
Hypertension is the leading attributable cause of death worldwide. According to the American Heart Association, a 5 mm Hg reduction in systolic blood pressure results in a 14 percent decrease in stroke, a 9 percent decrease in heart disease and a 7 percent decrease in overall mortality. Renal denervation has shown in published clinical studies to be safe, durable and effective in reducing systolic blood pressure by approximately 20 percent. Industry analysts suggest that there are more than 12 million patients worldwide whose blood pressure remains uncontrolled despite taking three or more anti-hypertensive medications, representing a global market opportunity for renal denervation that could ultimately grow to $30 billion.
Vessix will present interim clinical results from its pilot REDUCE-HTN clinical study for patients with uncontrolled hypertension at EuroPCR 2012, to be held in Paris May 15-18. Uta Hoppe, M.D., of Paracelsus Medical University in Salzburg, Austria, will present one-month post-treatment safety and efficacy data from patients treated at Paracelsus and additional patients treated at Georges Pompidou Hospital in Paris and OLV Ziekenhuis in Aalst, Belgium. The presentation will be made during the "Emerging Interventional Technologies for Treatment of Resistant Hypertension" session. Vessix will exhibit its V2 Renal Denervation System in booth M72.
For more information: www.vessixvascular.com


 November 14, 2025
November 14, 2025 









