November 26, 2019 — The University of Connecticut (UConn) Department of Kinesiology and Hartford Healthcare have selected the Medable Inc. cloud-based clinical trial platform to evaluate statin-related adverse events. It will use electronic patient-reported outcomes in a digital trial.
The collaborative digital study is intended to prospectively monitor participant response to statin medications via an app, and notify clinicians and patients when the patient should stop or start their medication based on presenting symptoms collected via electronic patient-reported outcomes (ePROs). The app seeks to use this methodology to identify statin-intolerant individuals in the study, by assessing the pattern, location and timing of their muscle symptoms.
Statins, such as Lipitor and Crestor are some of the most widely used drugs in the world, due to their effectiveness for reducing cholesterol and resultantly, cardiovascular disease (CVD) mortality and events. Although statins are widely available, cost-effective drugs, data suggests that over three years, patient compliance may be as low as 50 percent, due to side effects such as muscle pain. Improving statin adherence will thus have a powerful impact on mitigating the burden of preventable CV disease.
The statin-associated muscle symptom clinical index (SAMS-CI) is a method for assessing the likelihood that a patient’s muscle symptoms (e.g., pain, aching, cramping and weakness) are caused or worsened by statin use. This is important because the diagnosis and treatment of SAMS relies on patient self-reports of symptoms, which can be biased by a number of non-statin-related factors such as medications and health conditions.
“The problem with SAMS is that there are no diagnostic tests for this condition and clinicians have to rely on patient-reported perceptions of pain that may have occurred months or even years ago. We wanted to digitize the paper questionnaire into a clinical index onto an app, where patients could take their statin medication at home, and rank their pain levels in real-time. Medable was able to help us conceptualize the initial research idea into a patient-friendly app, and we wouldn’t have been able to do this without their guidance,” said Beth Taylor, Ph.D., lead study investigator and Associate Professor of Kinesiology at the University of Connecticut.
“Dr. Taylor’s research is critical to understanding the real-world effects of widely prescribed medications. It is important for the healthcare community to better understand which patient populations have adverse reactions to one of the most widely prescribed and important medications, statins. We are excited to partner with UConn on this groundbreaking research as it will benefit patients and highlights the power of digital to remove key barriers to access of clinical research,” said Michelle Longmire, M.D., CEO and co-founder of Medable.
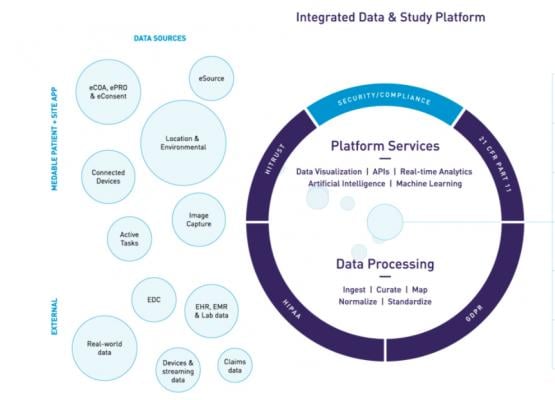
Medable is working on solutions to get effective therapies to patients faster by dramatically reducing the time from therapeutic development to market realization with digital data capture and real-time analytics. This can remove the complexities of clinical research to dramatically reduce trial timelines. The vendor is pioneering a new category of life science technologies that replace the stagnant and siloed data of traditional ePRO, eCOA, EDC, and eSource with an intelligent and unified end-to-end (E2E) platform for clinical trial execution. Medable’s end-to-end digital trials platform allows patients, healthcare providers, clinical research organizations and pharmaceutical sponsors to work together as a connected and empowered team in clinical trials and research.


 January 05, 2026
January 05, 2026 









