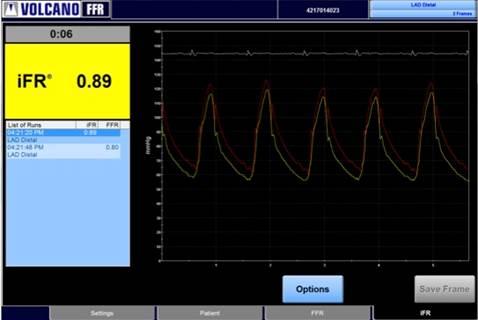
January 7, 2015 — Volcano Corp. announced that more than 1,000 systems have been activated with its instant wave-Free Ratio (iFR) Modality software, featuring a simplified workflow and reduced need for hyperemic agents.
The iFR Modality is a physiologic measurement performed using the same pressure wires and equipment utilized in cardiac catheterization labs for fractional flow reserve (FFR) but avoids injection of hyperemic agents into the patient that induce stress to the heart. This allows for a meaningful, lesion-specific assessment in seconds by amplifying the resting pressure waveform. The iFR Modality is used most efficiently with Volcano's Verrata Pressure Guide Wire, which is designed for simple disconnection and reattachment during a procedure and facilitates multiple quick measurements without the need for hyperemic agents.
The iFR Modality and other physiologic guidance tools including FFR and coronary flow reserve (CFR) are currently being studied in numerous prospective clinical trials as part of the DEFINE family of studies. These studies are designed to explore patient outcomes in a broad array of clinical presentations in more than 5,000 patients worldwide. The performance of the iFR Modality has been tested in approximately 800 patients as part of ADVISE II (Adenosine Vasodilator Independent Stenosis Evaluation), the first prospective, real-world registry comparing iFR and FFR. The iFR Hybrid Approach Analysis performed in the ADVISE II study demonstrated a statistically high correlation (sensitivity 90.7 percent for FFR less than or equal to 0.80, specificity 96.2 percent for FFR greater than 0.80). The hybrid method would have avoided the need to use a hyperemic agent in 65.1 percent of this patient population. Patients in ADVISE II were recruited from more than 40 centers in the United States and Europe, and all procedures were performed with operators blinded to the iFR values which were calculated offline at an independent core laboratory in Rotterdam, the Netherlands.
"We chose to evaluate the hyperemic savings in our own subset of patients and have been very pleased with iFR," commented Vincent J. Pompili, M.D., FACC, director, Interventional Cardiovascular Medicine and Cardiac Catheterization Laboratories at The Ross Heart Hospital at The Ohio State University Wexner Medical Center. "In our subset of patients thus far, we've seen more than a 60-percent reduction in the need for hyperemic agents using the iFR Hybrid Approach, which is consistent with the ADVISE II study. Our patients appreciate not having to receive a hyperemic agent but still receive the benefits of intracoronary physiologic measurements. So far I have been impressed with the ease of use, and repeatability of the software."
For more information: www.volcanocorp.com


 November 14, 2025
November 14, 2025 









