September 12, 2014 — Volcano Corp. announced it will be launching its interventional precision guidance tools at the 27th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium, being held in Washington, D.C., Sept. 13-17.
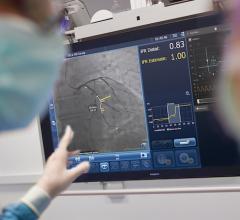
Enhancing coronary imaging, the SyncVision co-registration system will be shown at TCT for the first time since its limited market release. SyncVision features a live, online image processing workstation for coronary catheterizations that allows physicians to simultaneously navigate on both an angiogram and an IVUS (intravascular ultrasound) image in a single correlated view. Attendees who visit the booth will experience hands on how SyncVision is designed to combine the functionality of the angiographic road map with the precision of the intra-vessel IVUS image. The product is currently installed in select institutions in the United States, Europe, Japan and China and will be featured in case demonstrations during the scientific sessions.
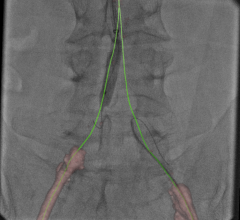
As a first for Volcano, the SyncVision ‘Angio Plus’ suite of tools not only enhances the IVUS image, but augments the angiogram itself by offering image enhancement and stabilization tools. Physicians no longer have to use a Volcano IVUS catheter or pressure wire to augment the precision of the angiogram.
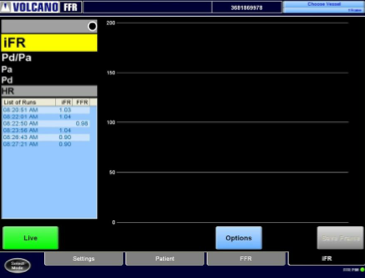
The company also announces the full market release of its proprietary iFR modality in Meet the Expert sessions at its booth and live case transmissions. The iFR modality is a physiologic measurement performed using the same pressure wires and equipment utilized in cath labs for fractional flow reserve (FFR), which avoids injection of hyperemic agents into the patient that can induce stress to the heart. This allows for a meaningful, lesion-specific assessment in seconds by amplifying the resting pressure waveform. The technology has been studied in more than 4,000 patients, was cleared by the U.S. Food and Drug Administration (FDA) in March 2014 and is now installed in more than 700 hospitals in all major geographies.
"Volcano will continue to be present for physicians and for patients, providing more precise tools to guide these important therapies," said Scott Huennekens, president and CEO of Volcano Corp. "We believe the addition of the iFR modality and SyncVision co-registration tools will allow us to add precision to everyday tools, with workflows that are fast and simple enough to be adopted in the busiest of cath labs. TCT is a great week for physicians and hospital team members to see these tools live for themselves and plan how to make them a part of their cath lab routine."
For more information: www.volcanocorp.com

 January 18, 2022
January 18, 2022