
The DEFINE GPS study will be the first time that the use of iFR in conjunction with an angiography image-guided co-registration system for PCI guidance and the optimization.
February 12. 2020 — Philips announced a new randomized controlled trial to assess patient outcomes after receiving a percutaneous coronary intervention (PCI) guided by Philips’ co-registration platform compared with today’s standard of care treatment guided by an angiogram alone. The Philips platform combines data of an instant wave-free ratio (iFR) measurement (an easier to use fractional flow reserve (FFR) technology) and the interventional X-ray angiogram image.
European and U.S. guidelines already endorse the use of coronary physiologic measurements with iFR and FFR for diagnostic purposes to determine the significance of a narrowed coronary artery. The DEFINE GPS (Distal Evaluation of Functional performance with Intravascular sensors to assess the Narrowing Effect: Guided Physiologic Stenting) study will be the first time that the use of iFR in conjunction with the Philips Image-Guided Co-registration System (SyncVision) is evaluated for PCI guidance and the optimization of treatment outcomes.
PCI is an image-guided, minimally invasive treatment to open a coronary artery blockage that is causing a reduced blood flow (ischemia) to heart tissue. Under the current standard of care, clinicians navigate a balloon catheter and coronary stent to the treatment area using coronary angiography. In the study, an iFR pullback measurement, which uses pressure wires to map the pressure profile of the disease distribution along the length of the affected vessel, will be overlaid on the X-ray image to guide treatment. iFR is a next-generation physiologic measurement that uses the same pressure guide wires and equipment as FFR but avoids the administration of hyperemic agents to patients.
“PCI has made a major positive impact on many coronary artery disease patients’ lives,” said Allen Jeremias, M.D., U.S. and principal investigator of the DEFINE GPS study, from St. Francis Hospital, Roslyn, N.Y., and Cardiovascular Research Foundation, New York. “However, when we look back at all the major, high-quality stent trials over the past 20 years we see that around 20-30% of patients continue to have recurring chest pain at one year after receiving treatment. With the DEFINE PCI study we observed that the current approach to PCI has limitations for identifying the locations of physiologically significant arterial lesions. With DEFINE GPS we will be able to determine if a physiology-based PCI approach results in superior patient outcomes when compared with standard angioplasty.”
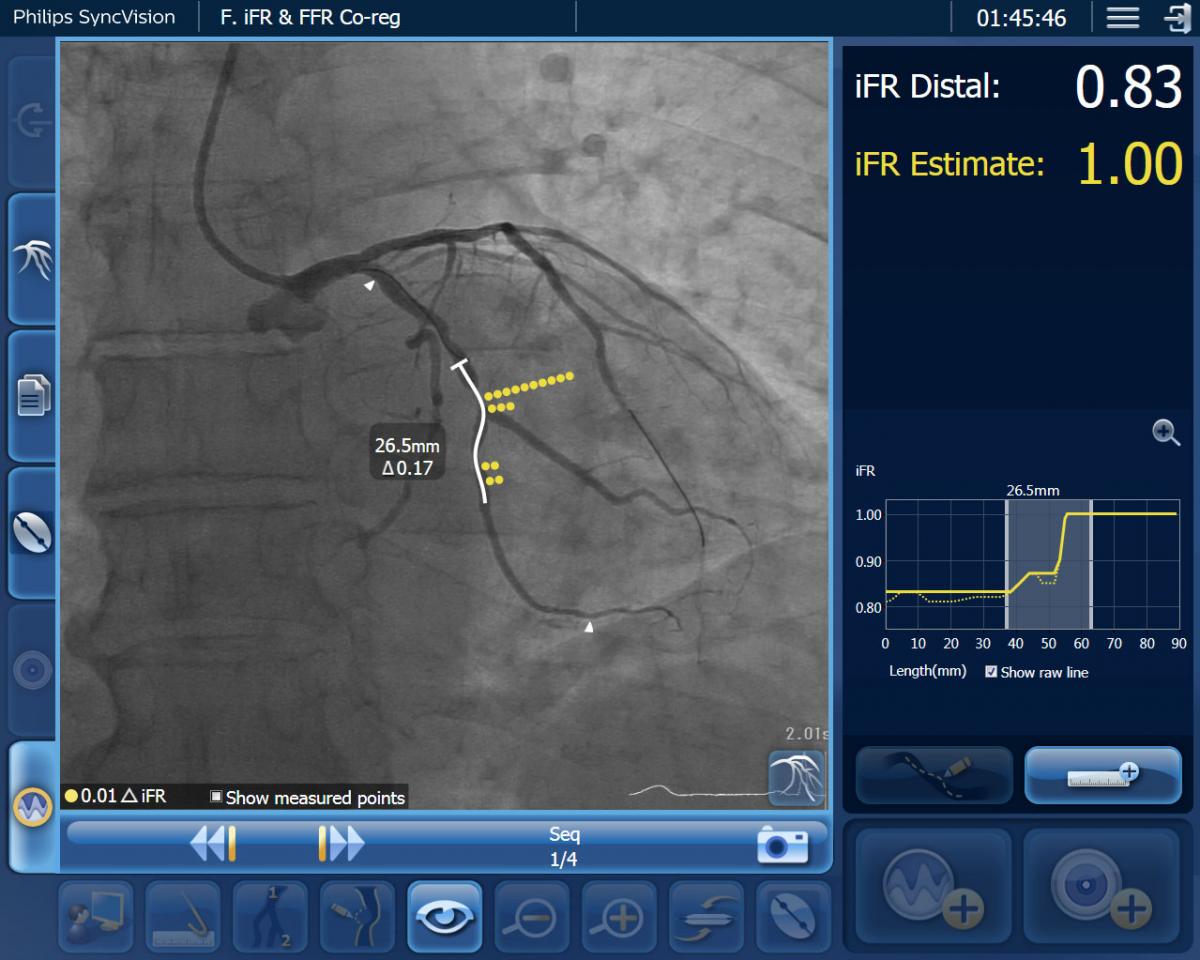
A view of the Philips SyncVision technology being used in the DEFINE GPS study, which overlays iFR co-registed with the angiographic image of the coronaries. It uses a series of yellow dots to note drops in blood flow pressures in the vessel segment and their severity. This can be used to help pinpoint culprit lesions and define stent lengths needed.
“As coronary stenting is applied to increasingly complex patients, it is essential that we ensure that all segments of coronary artery disease that need treatment are treated, a process that we believe can be facilitated by iFR assessment of the entire coronary artery, co-registered to the angiogram.” said Gregg W. Stone, M.D., chairman of the DEFINE GPS trial, director of academic affairs for the Mount Sinai Heart Health System, professor of medicine (cardiology) and professor of population health sciences and policy at the Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York. “We are thrilled to be able to examine the extent to which this technique improves patient outcomes in the large-scale DEFINE GPS trial.”
The global multicenter, prospective, randomized controlled DEFINE GPS study will investigate the impact of iFR Co-registration on both outcomes and cost effectiveness. The primary endpoint is target vessel failure (a composite of cardiac death, target vessel Myocardial Infarction and ischemia-driven target vessel revascularization) or rehospitalization for progressive or unstable ischemia at two years.
The Philips Image-Guided Co-registration System (SyncVision) is part of Philips’ portfolio of systems, smart devices, software and services in image-guided therapy, which combine to provide healthcare providers with sophisticated, procedure-oriented solutions.
The DEFINE GPS study is sponsored by Philips with the Cardiovascular Research Foundation overseeing core lab and clinical event committee activities. The first patients will be recruited in the second half of 2020.
Related iFR, FFR and PCI Guidenace Technology Content:
How Interventionalists Can Keep From Missing Coronary Stenoses
Updated SCAI Expert Consensus Says Either FFR, iFR Can Be Used
VIDEO: iFR Equal to FFR Outcomes in Coronary Lesion Evaluation — Interview with Justin Davies, MBBS, and Matthias Götberg, M.D.
New Technology Directions in Fractional Flow Reserve (FFR)
iFR More Cost-Effective Than FFR in PCI Guidance, DAIC
Easier to Use iFR Equal to Outcomes of FFR in Coronary Lesion Evaluation
VIDEO: iFR Found More Cost Effective Over Standard FFR — Interview with Manesh Patel, M.D.
One in Four Patients Have Residual Ischemia Following PCI
References:


 October 24, 2025
October 24, 2025 









