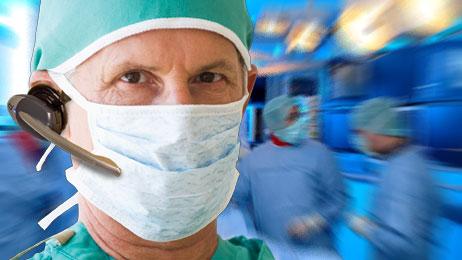
March 6, 2014 — Memorial Hospital in Carbondale, Ill., has chosen Quail Digital’s healthcare wireless headset system. It will be used in catheterization and electrophysiology labs where all coronary and peripheral vascular interventions, device implants and electrophysiology procedures are performed.
Quail Digital wireless headsets are worn by all staff, including physicians, to ensure everyone is kept in the communication loop during procedures. The Quail Digital system’s hands free, digital design ensures ease of use with excellent audio quality, even with multiple systems being used in the same facility.
Nine headsets were installed for use in four catheterization and electrophysiology labs.
Mike Bull, cath lab manager, said, “Our teams like the headsets and we’ve found that communication has improved during our procedures thanks to the system. It is now much easier for our staff monitoring the procedure from the control to hear what is happening in the lab, and their documentation is more accurate and thorough as a result. The headset system has been a good investment and we are very happy with the improved communication and the associated benefits.”
Outside healthcare, brands using Quail Digital systems include: IKEA, The Co-operative, Sainsbury’s, McDonald’s, KFC, Burger King, Sonic, Popeyes, Edeka, Bauhaus, Starbucks, Taco Bell, Gap.
For more information: www.quaildigital.com


 November 14, 2025
November 14, 2025 









