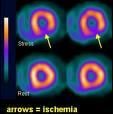
December 31, 2008 – According to results from a Phase 2 clinical study (BP-23), Zemiva, when combined with the standard of care for the diagnostic evaluation of the chest pain patient, improved sensitivity for the detection of cardiac ischemia by more than 50 percent (p
The study showed that Zemiva’s sensitivity was more than 50 percent greater (p
Zemiva is a fatty acid analog also known as 123I-BMIPP or Iodofiltic Acid I 123 that detects cardiac ischemia by revealing abnormalities in the fatty acid metabolism of the heart. Under normal conditions, 70 to 80 percent of the energy for the heart is produced by the metabolism of fatty acids. However, in ischemic conditions where there is a lack of oxygen, fatty acid metabolism is drastically reduced and carbohydrates become the heart’s primary energy source. This shift in metabolic activity persists for some time, and the phenomenon, called ischemic memory, has been shown by Zemiva imaging to persist for at least 30 hours after chest pain has subsided. Zemiva can be imaged using standard nuclear medicine cameras.
The trial enrolled 510 patients over 14 months at 50 hospitals throughout North America. The primary objective was to evaluate the ability of Zemiva to identify myocardial ischemia in patients who present to the emergency department with suspected acute coronary syndrome. The primary endpoint of the trial was to determine the performance (sensitivity and specificity) of Zemiva and the key secondary endpoint was to determine the clinical benefit of the use of Zemiva as a complement to standard of care. The trial met both the primary and key secondary endpoints. Zemiva was well tolerated. There were no serious adverse effects associated with the product and no patients discontinued the product due to adverse events.
These results were consistent for the subset of patients with acute coronary syndrome (ACS). In both cases, sensitivity and negative predictive value was improved while specificity was maintained. In patients with a negative Zemiva scan, there were no hard cardiac events, including myocardial infarctions or death from cardiac causes during the 30-day follow up.
For more information: www.molecularinsight.com


 August 17, 2023
August 17, 2023 








