May 5, 2009 - The Society of Cardiovascular Angiography and Interventions (SCAI) will highlight the Cardiac Dimensions Inc. AMADEUS study during its 32nd annual scientific sessions in Las Vegas, Nev., later this week.
AMADEUS (CARILLON Mitral Annuloplasty Device European Union Study) was a 30 patient safety and performance study of the CARILLON Mitral Contour System conducted at six centers in Germany, Poland, and the Netherlands. The study met its primary safety endpoint and its secondary performance endpoints both at six months. The results are scheduled for presentation Friday, May 8 by Prof. Tomasz Siminiak, M.D., from the Poznan University of Medical Sciences, Cardiac and Rehabilitation Hospital in Poznan, Poland. Dr. Siminiak was the leading enroller in the AMADEUS study and has implanted the most CARILLON implants.
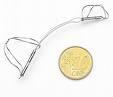
The CARILLON system is designed for percutaneous treatment of functional mitral regurgitation (FMR). FMR results when the valve leaflets are pulled apart by the growing heart and do not close properly. This leads to a significant reduction in the volume of oxygenated blood channeled from the heart to the rest of the body. Since 85 percent of heart failure patients experience heart enlargement, FMR is a common condition among these patients. FMR Patients are short of breath, fatigued, and experience a significant reduction in their quality of life as they become less and less mobile. The CARILLON system may offer a minimally invasive alternative to these patients who, up until now, have had limited treatment options. The CARILLON system is delivered via a catheter inserted through the jugular vein and guided into the heart. The implant is placed around the valve and is tightened in order to facilitate proper closing of the mitral valve leaflets.
“I have been extremely impressed with the safety, simplicity and performance of the CARILLON Mitral Contour System,” Dr. Siminiak said. “In my experience, many of my patients have benefited from this new therapy. I am pleased that SCAI has chosen to recognize AMADEUS. The clinical results obtained have been very intriguing, which in turn create enthusiasm that this might become a true breakthrough treatment for a large group of patients who really have had no other option. My hope is that my colleagues who review the study results become interested in participating in further clinical and commercial use of this system.”
The CARILLON Mitral Contour System combines a proprietary implantable device and delivery system. The implant consists of a shaping ribbon between distal and proximal anchors. It is delivered percutaneously via jugular vein access under fluoroscopic guidance. The implant is designed to be positioned, adjusted and gently anchored in the coronary sinus/great cardiac vein to reshape the annulus around the mitral valve, thereby reducing mitral regurgitation. Preclinical and early clinical data have suggested both a reduction in mitral regurgitation and improvements in other key parameters including NYHA class, six minute walk distances, and quality of life.
For more information: www.cardiacdimensions.com


 January 05, 2026
January 05, 2026 









