October 9, 2009 – Clarian Cardiovascular has established the Midwest’s only Transradial Center of Excellence in an effort to offer patients a catheterization procedure that significantly reduces recovery time and improves patient outcomes using transradial cardiac catheterization instead of femoral access.
Transradial cardiac catheterization uses the wrist, not the groin, for catheter insertion. While this technique is not new, less than 2 percent of cardiac catheterizations in the United States use the transradial approach because only a small percentage of interventional cardiologists are trained in this specialized procedure. In fact, transradial access leads the way in many parts of the world, including Europe, Japan, China and Canada.
Traditionally, cardiac catheterization uses the femoral artery in the right groin as the point of insertion for the catheter. This entry point is often difficult to access and may be hard to compress after the procedure to stop the bleeding. The recovery time for the femoral approach is 20 minutes of pressure followed by six to eight hours of bed rest to allow the hole in the groin to heal.
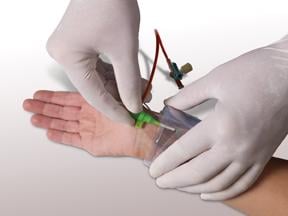
The transradial catheterization is performed using an artery in the wrist. This technique is safer, offers no scarring, a decreased risk of bleeding, lowered risk of all complications, less risk of trauma to adjacent nerves and blood vessels, reduced hospital and health care costs and a shorter recovery period. The recovery time for the transradial approach is less than two hours bed rest and discharge within four hours depending on the procedure.
Transradial cardiac catheterizations benefit all patients but especially those with low back pain, arthritis, obesity and peripheral vascular disease.
“The femoral approach remains the dominant technique because it is easy to learn. However, this common approach leads to substantial patient discomfort due to prolonged groin compression resulting in immobilization,” said George Revtyak, M.D., FACC, FSCAI, FAHA, Clarian Cardiovascular. "That’s why it was important for Clarian Cardiovascular to establish the Transradial Center of Excellence so people in the state of Indiana and beyond have access to another cardiac catheterization option that is truly beneficial in more ways than one.”
The Clarian Transradial Center of Excellence will also offer a physician training center with state-of-the-art equipment at Methodist Hospital where cardiologists will receive hands-on training covering the clinical and practical aspects of transradial access, including the use of simulators to focus on the use of different catheters and their tactile feel. The 3D component of the simulator allows physicians to truly understand how catheters respond to torque and steering.
The transradial catheterization technique was pioneered by a Dr. Lucien Campeau, a French-Canadian physician, in 1989, and in August 1992, the first patients were treated with this procedure at the Amsterdam Department of Interventional Cardiology of the Onze Lieve Vrouwe Casthuis in Amsterdam.
Clarian Health is an Indiana-based, private, nonprofit organization offering a broad base of tertiary services, specialized pediatric care and a Level 1 Trauma Center. Clarian is Indiana's most comprehensive health center and one of the busiest hospital systems in the nation. Clarian owns or is affiliated with 18 hospitals and health centers throughout Indiana, including Methodist and Indiana University Hospitals, Riley Hospital for Children, Clarian West Medical Center and Clarian North Medical Center. Clarian Health is also affiliated with the Indiana University School of Medicine. Clarian Health also operates the Methodist Hospital, Indiana University Hospital and Riley Hospital for Children campuses.
For more information: www.clarian.org


 November 14, 2025
November 14, 2025 









