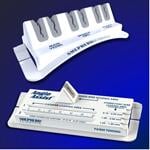
November 9, 2010 – To assist interventionalists with organizing, securing and loading interventional tools during catheterization procedures, Vascular Solutions offers the Angio Assist Docking Station and Teirstein Edge Device Organizer.
The Angio Assist is an easy-to-use docking station that facilitates the introduction of guidewires into interventional devices. Side clips provide rapid, secure attachment to the operative field and a user-friendly design features a device loading area, which facilitates the alignment and introduction of 0.014-inch guidewires into catheters and atherectomy burrs. Additionally, a triangular guidewire slit secures the in-place guidewire, simplifying single-operator procedures.
The Teirstein Edge is a device organizer designed to neatly organize guidewires and catheters during interventional procedures. Composed of six proprietary, triangular friction fit slits that secure guidewires and catheters, the Teirstein Edge allows the operator to make controlled, micro movements of catheters and wires, or to tightly lock devices in place, thereby minimizing guidewire loss during the procedures. The Teirstein Edge easily attaches to the sterile drape through unique side-clips, and also features vessel identification nomenclature, separating main vessel, branch one and branch two locations.
Designed and manufactured by Shepherd Scientific in La Jolla, Calif., the Angio Assist and Teirstein Edge were developed to overcome everyday challenges during interventional procedures. The sterile, single use Angio Assist and Teirstein Edge devices are available in the United States exclusively through Vascular Solutions and internationally through Shepherd Scientific.
For more information: www.vascularsolutions.com or www.shepherdscientific.com


 November 14, 2025
November 14, 2025 









