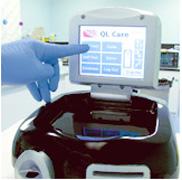
February 7, 2011 – Final testing for a point-of-care blood analyzer will begin at four hospitals in North America. The QL Care (QLCA) analyzer, by CardioGenics, tests for troponin-I levels, and data will be used to file a 510(k) application with the U.S. Food and Drug Administration (FDA).
The company will use the Siemens ADVIA Centaur Analyzer and its related troponin-I test as the reference standard for its clinical testing. The goal of this comparative testing is to confirm that the results from the QL Care Analyzers and the Siemens ADVIA Centaur systems are equivalent. CardioGenics expects to begin enrolling patients by May 2011, upon approval of the Institutional Review Boards.
The comparative trials will take approximately 45 days to complete. Final testing is expected to start during the early third-quarter of 2011 and be completed two months later.
“Our goal has always been to create test products at the point of care that compare favorably to the large and expensive lab-based machines," said Yahia Gawad, M.D., CEO of CardioGenics.
For more information: www.cardiogenics.com


 January 05, 2026
January 05, 2026 









