June 14, 2011 – An initial animal study of a next-generation cardiac assist device for heart failure patients using a transcutaneous power supply has been completed. The C-Pulse Heart Assist System system made by Sunshine Heart Inc., is designed for class III/ambulatory class IV heart failure patients.
A study conducted at Texas Heart Institute in Houston, demonstrated that a combined driver and C-Pulse cuff implanted around the heart's aorta can be successfully powered from a wireless, external battery unit. This fully-implantable C-Pulse model would eliminate the need for wires to breach the skin, thereby improving patient comfort and reducing the risk of infection.
"There are hundreds of thousands of people with early heart failure that could benefit from a fully implantable, untethered device that is safe and easy to implant and that doesn't compromise patient mobility," said William Cohn, M.D., director of minimally invasive surgery at Texas Heart Institute. "The promising outcomes of this study could eventually lead to an important therapy that has the potential to change the early management of heart failure, which remains our biggest unmet health care challenge in the United States today."
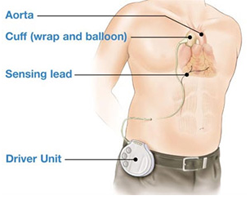
The C-Pulse system is an investigational device designed to relieve the symptoms of heart failure, such as shortness of breath and reduced mobility, through the use of counter-pulsation technology. A cuff placed around the outside of the ascending aorta (just above the aortic valve) inflates and deflates in synchronization with the heartbeat, potentially improving the efficiency of the heart, increasing coronary blood flow, and reducing the heart's pumping load. Currently, both the C-Pulse power unit and driver unit are located outside the body and connect to the cuff via tubing that exits through the patient's abdomen.
In the recently tested, fully implantable C-Pulse system, the power source remained outside the body. The driver, which controls the C-Pulse cuff, was miniaturized and implanted inside the animal model. The internal driver was powered through the skin using transcutaneous energy transfer (TET), an established technology that uses a high-frequency electromagnetic field to transfer electricity through the skin without wires. The system successfully augmented the animal's heart function, confirming the viability of a fully implantable unit.
"Our goal is to ultimately develop a fully implantable device that can connect directly to a patient's pacemaker," said Dave Rosa, Sunshine Heart's CEO.
The C-Pulse Heart Assist System recently completed enrollment of 20 patients in its U.S. Food and Drug Administration (FDA)-approved investigational device exemption (IDE) feasibility study. The study was primarily designed to assess safety and provide indications of performance of this device in moderate to severe heart failure patients who suffer from symptoms such as shortness of breath and reduced mobility. Once the six-month follow-up with the 20th patient is completed, Sunshine Heart will submit the feasibility data to the FDA. Shortly thereafter, the company will seek FDA approval for the pivotal trial protocol.
The FDA-approved IDE feasibility study is available to men and women between the ages of 18 to 75 who suffer from class III/ambulatory class IV heart failure and for whom standard drug therapy has failed.
For more information: www.sunshineheart.com


 January 05, 2026
January 05, 2026 









