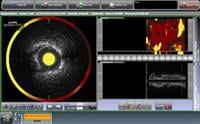
July 13, 2011 – InfraReDx Inc. announced enrollment of the first patient in its Phase 2 clinical trial, CANARY (Coronary Assessment by Near-infrared [NIR] of Atherosclerotic Rupture-prone Yellow). CANARY is designed to test the hypothesis that NIR-guided use of an embolic protection device (EPD) filter during percutaneous coronary intervention (PCI) can reduce the rate of periprocedural heart attacks in patients identified as having high-risk lipid core plaques (HR-LCPs). InfraReDx’s LipiScan IVUS system is the only multimodality coronary imaging device approved in both the United States and Europe. It is in routine clinical use to detect the LCPs known to complicate stenting and suspected to cause most heart attacks.
Multiple studies indicate that approximately 10 percent of patients undergoing PCI experience a heart attack during the stenting procedure. There is considerable clinical evidence indicating that such heart attacks are caused by disruption by balloon dilation of large LCPs. The contents of these plaques are then carried downstream and occlude the small coronary arteries. LipiScan IVUS can detect the large LCPs most likely to cause this problem, and thereby identify cases in which a distal protection filter might be most effective.
“We will test the hypothesis that LipiScan IVUS, by detecting large lipid core plaques that are prone to embolization when dilated, can identify the subset of coronary patients in which a device designed to prevent distal embolization will be effective,” said Gregg W. Stone, M.D., professor of medicine, director of cardiovascular research and education, Center for Interventional Vascular Therapy, Columbia University Medical Center, and principal investigator of CANARY. “A positive result of CANARY will be a step forward in efforts to enhance the safety of coronary stenting.”
CANARY is a prospective, multicenter, randomized, open-label Phase 2 trial expected to enroll 108 patients at 10 clinical sites in the United States. Patients identified as having a HR-LCP are randomized 1:1 to standard treatment (angioplasty and stent implantation) or standard treatment plus the use of a filter designed to prevent embolization. Subjects whose target lesion does not contain HR-LCP are assigned to standard therapy; these subjects are not randomized.
The primary efficacy endpoint in the study is reduction in peri-procedural MI, which is defined as an elevation of cardiac biomarkers. The primary safety endpoint is the occurrence of adverse events collected between discharge and one year post-procedure.
Boston Scientific is providing the FilterWire EZ embolic protection system for the study.
For more information: www.infraredx.com


 January 05, 2026
January 05, 2026 









