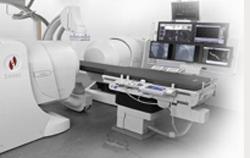
January 12, 2012 – Stereotaxis Inc. (announced the completion of the first 50 clinical procedures using the company's new Niobe ES system to treat patients with a variety of complex cardiac arrhythmias. A majority of the first 50 cases were performed to treat atrial fibrillation (AF), the most common type of cardiac arrhythmia. Positive initial results with the Niobe ES system in Europe demonstrate that the average time for completion of mapping and ablation for the initial AF patients was 69 minutes. The data will be featured at the Boston Atrial Fibrillation Symposium 2012 to be held on January 12-14, 2012.
The Epoch platform, which encompasses the Niobe ES system, is the company's new generation comprehensive solution for the electrophysiology (EP) laboratory. It is designed to improve efficiency with a fully remote, networked and modular robotic, magnetic system that enables greater surgical precision and improved catheter control while reducing the risk of complications. Stereotaxis began initial shipments of the Epoch platform in mid-December 2011, including six system upgrades from the Niobe II navigation system at leading medical centers in the United States and Europe.
Professor Carlo Pappone of Villa Maria Cecilia Hospital, Cotignola Italy, said, "My vision was to click on the map and for the catheter to quickly and precisely move to that spot. Today with the Epoch platform, this is a reality. I believe the Epoch platform is one of the most important innovations for the EP practice to date. With the Epoch technology all physicians can successfully and consistently perform high quality AF procedures with the assurance of superior patient care."
The Heart Hospital Baylor Plano in Plano, Texas was the first North American site to install the new Epoch platform, and the first hospital in the world to perform an EP procedure using the new system.
"Interventional physicians want to leverage advanced technology that minimizes surgical risks to the patient while increasing the likelihood of a favorable outcome," said Brian DeVille, M.D., FACC, eletrophysiologist on the medical staff at The Heart Hospital Baylor Plano. "The new Niobe ES system will enable electrophysiologists on the medical staff to deliver therapy in a precise manner, while reducing X-ray exposure and procedure time for our patients."
"The initial feedback and interest in our new Epoch platform has been very favorable," said Michael P. Kaminski, president and CEO of Stereotaxis. "We look forward to continuing to build on the momentum of this milestone and have commitments for 12 additional system upgrades to Niobe ES which will be installed over the next few months."
For more information: www.stereotaxis.com, www.odysseyexperience.com


 January 05, 2026
January 05, 2026 









