May 16, 2013 — Sorin Group has received U.S. Food and Drug Administration (FDA) approval of its Investigational Device Exemption (IDE) application and clinical trial protocol to begin evaluating its Perceval S sutureless aortic tissue valve.
Rakesh Suri, M.D., associate professor of surgery, consultant cardiovascular surgeon, Mayo Clinic, Rochester, Minn., is the principal investigator for the PERCEVAL S IDE trial that will involve up to 25 U.S. centers. The purpose of this prospective, non-randomized, multi-center clinical trial is to demonstrate the safety and effectiveness of the Perceval S sutureless heart valve when used to replace a diseased or dysfunctional aortic valve or aortic valve prosthesis.
Aortic stenosis is a degenerative disease resulting from a progressive age-dependent buildup of calcium that disrupts blood flow across the aortic valve.
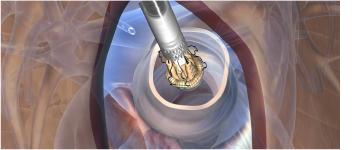
Perceval S is a bioprosthetic valve designed to replace a diseased native or malfunctioning prosthetic aortic valve in patients with aortic stenosis using either traditional or minimally invasive heart surgery. Due to its original characteristic of allowing sutureless positioning and anchoring at the implantation site, the Perceval S aortic valve will offer significant advancements in surgical Aortic Valve Replacement (AVR) technology.
Perceval S is designed to be implanted through either a traditional open heart surgical approach or through a Minimally Invasive Cardiac Surgery (MICS) partial sternotomy or right mini-thoracotomy implantation technique. MICS techniques are increasingly important for AVR because they reduce surgical trauma and morbidity, which is an important consideration for the growing number of AVR patients.
For more information: www.sorin.com


 January 05, 2026
January 05, 2026 









