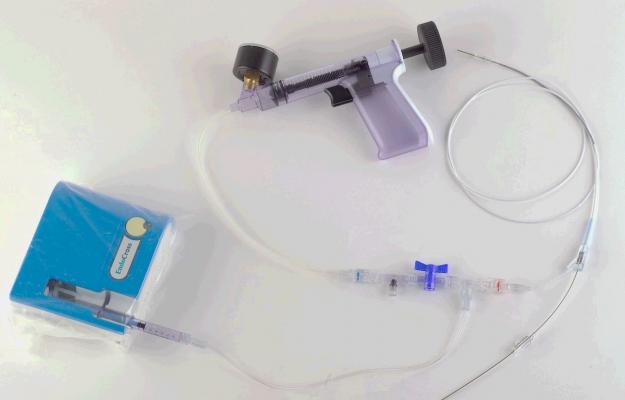
Enabler-C Catheter System
October 3, 2013 — EndoCross, Ltd. announced today that it has already enrolled one-third of the patients planned for the first-in-human study of its Enabler-C Catheter System for Crossing Coronary Chronic Total Occlusions (“Enabler 3C” study). The Enabler 3C clinical study is taking place at the Institut Cardiovasculaire Paris-Sud under principal investigator Dr. Yves Louvard.
The Enabler 3C study is investigating the safety and performance of the Enabler-C Catheter System in facilitating guidewire advancement through chronic total occlusions in the coronary arteries. Early results are demonstrating significant crossing capability with no adverse effects. It is important to note that the same technology is already CE-marked and has been used successfully in 22 medical centers around the world for the treatment of CTO in the lower limbs.
“The Enabler technology is designed to assist in the controlled advancement of guidewires through the most challenging arterial lesions. Our CTO crossing system is unique in its ability to track the vessel without the need for direct physician navigation of the wire,” said Yaron Eshel, Chief Operating Officer of EndoCross.
“The Enabler is currently the only crossing device that spontaneously adjusts the wire position, thus helping to maintain luminal positioning. While other companies have focused on providing additional energy to the tip of the wire, the Enabler uses cyclical and controlled incremental advancements to guide the wire through the totally occluded vessels,” Mr. Eshel added. “The Enabler’s progress through the vessel does not require additional imaging or capital equipment. In addition, the system is familiar to the physician as it is based on traditional balloon catheter technologies.”
“Compared to failed CTO PCI, successful CTO PCI has been associated with improvement in angina, left ventricular function, and increased survival,” said Mr. Eshel. Despite these benefits, CTO PCI is performed infrequently, most likely due to historically low success rates, technical complexity, and the potential for major procedural complications(1). “The use of the Enabler-C Catheter System may overcome these drawbacks and foster improved patient outcomes,” he added.
For more information, visit: http://endocross.eu/
References
[1]Rosamond W, Flegal K, Furie K, et al; Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008;117(4):e25


 January 05, 2026
January 05, 2026 









