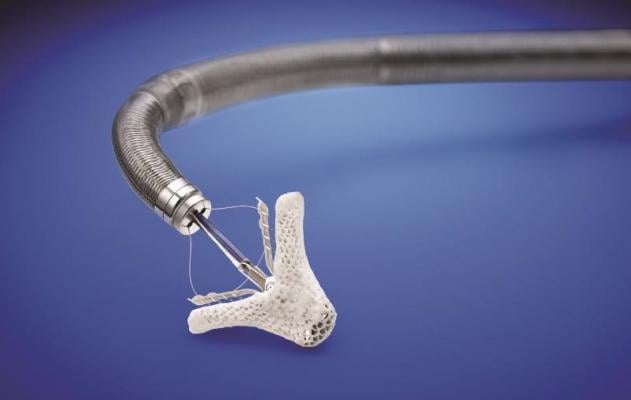
October 25, 2013 — The U.S. Food and Drug Administration (FDA) has granted market clearance for the MitraClip, the first minimally invasive transcatheter device to repair mitral regurgitation (MR). The device is first of its kind to gain approval for use in patients in the United States and part of a new movement away from open heart surgical repair toward minimally invasive procedures performed in a cath lab or hybrid operating room.
Abbott said it plans to launch the device immediately in the U.S. market. The MitraClip device has been approved for patients with significant symptomatic degenerative MR who are at prohibitive risk for mitral valve surgery. Degenerative MR is caused by an anatomic defect of the mitral valve of the heart. Prohibitive risk is determined by the clinical judgment of a heart team due to the presence of one or more documented surgical risk factors.
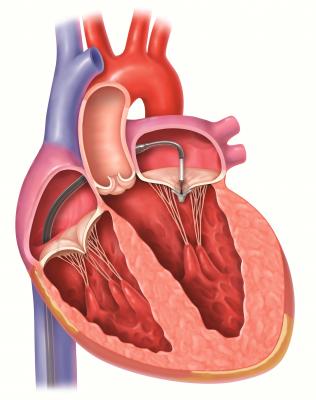
MR is a debilitating, progressive and life-threatening disease in which a leaky mitral valve causes a backward flow of blood in the heart. The condition can raise the risk of irregular heartbeats, stroke and heart failure, which can be deadly. Mitral regurgitation is common, affecting more than 4 million Americans — nearly one in 10 people aged 75 and above.[1] Open heart mitral valve surgery is the standard-of-care treatment, but many patients are at prohibitive risk for an invasive procedure. Medications for the condition are limited to symptom management and do not stop the progression of the disease.
"As cardiac surgeons, we see patients with severe mitral regurgitation who we can technically operate on but who are just too frail or sick to survive mitral valve surgery with a reasonable risk and quality of life," said Michael Mack, M.D., director of cardiovascular research and cardiovascular medicine and director of cardiovascular surgery at Baylor Health Care System in Dallas. "With the MitraClip system, heart teams now have a catheter-based, less-invasive treatment option that can help patients who cannot withstand surgery regain their quality of life."
Multiple trials, published reports and registries of patients treated with the MitraClip device consistently demonstrate a positive safety profile, reduction in mitral regurgitation, improvement in symptoms and reduction in hospitalizations for heart failure, even in some of the most ill and debilitated patients. More than 11,000 patients in more than 30 countries have been treated with the MitraClip device.
"MitraClip is a breakthrough in the treatment of severe mitral regurgitation, a condition that is progressive and causes extreme fatigue and shortness of breath, eventually making even simple tasks virtually impossible, and increasing the risk of stroke, heart failure and death," said Ted Feldman, M.D., FSCAI, director, cardiac catheterization laboratory and The Mr. and Mrs. Charles R. Walgreen Chair in Interventional Cardiology, NorthShore University Health System, Evanston, Ill. "Clinical data and real-world international experience, dating back to 2003, have consistently shown that the MitraClip is a safe and effective therapy for patients unable to undergo mitral valve surgery, providing meaningful improvements in quality of life that are sustained over time. It has allowed many of my patients to go from bed rest to a more active lifestyle shortly following treatment."
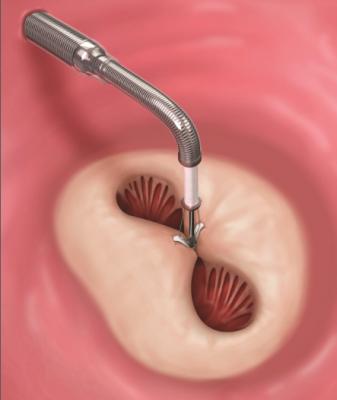
Abbott's MitraClip repairs the mitral valve without the need for an invasive surgical procedure. The device is delivered to the heart through the femoral vein, a blood vessel in the leg, and once implanted, allows the heart to pump blood more efficiently, thereby relieving symptoms and improving patient quality of life. Patients undergoing MitraClip treatment typically experience short recovery times and short hospital stays of two to three days.[2]
"FDA approval of MitraClip marks an important milestone for Abbott as we continue to bring forward innovative therapies to help patients live better lives," said Chuck Foltz, senior vice president, vascular, Abbott. "We look forward to making this technology available to specialized centers in the U.S. with multi-disciplinary teams experienced in the management of patients with heart valve disease, a model that facilitates dialogue across physician specialties and provides patients with the best treatment outcomes."
Abbott continues to conduct clinical trials of the MitraClip therapy through two landmark, prospective, randomized trials — COAPT in the United States and RESHAPE-HF in Europe — that will evaluate the impact of MitraClip treatment on the progression of heart failure. The studies also will generate important clinical and economic data that may support development of treatment guidelines, expanded indications and reimbursement. Both studies are currently enrolling patients.
A New Path in Heart Valve Repair
The MitraClip is the third transcatheter heart valve repair system cleared in the United States. Medtronic’s Melody pulmonic valve was the first transcatheter valve cleared for humanitarian use in the United States in January 2010. The Edwards Lifesciences Sapien transcatheter aortic valve replacement (TAVR) system was cleared for the U.S. market in November 2011. Medtronic’s CoreValve TAVR system is currently in FDA trials and at least 30 other companies are developing transcatheter repair or replacement systems for the aortic and mitral valves.
These devices are currently used for very sick patients who would otherwise not be considered eligible for open heart surgical valve repair because of concerns over survival. However, experts believe that as these devices are shown to be as effective and durable in trials where they are implanted in increasingly less sick patients, transcatheter valve repair will eventually replace the open surgical repair.
For more information: www.abbott.com
References: