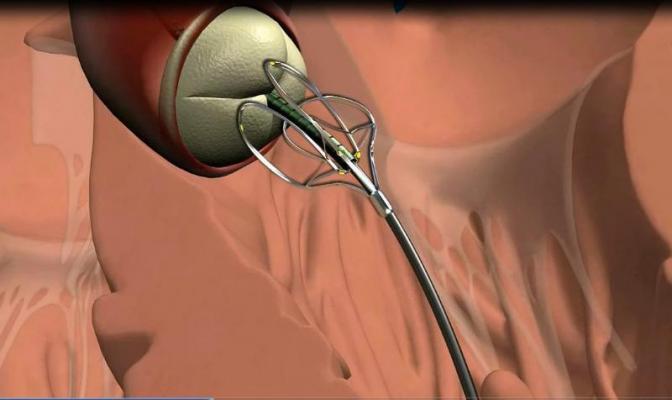
January 14, 2014 — MediValve Ltd. announced that it has received clearance of a Pre-Marketing Notification Application (510(k)) from the U.S. Food and Drug Administration (FDA) for its acWire
Guidewire. The acWire is intended for use in peripheral vascular and heart
catheterization procedures to introduce and assist in positioning diagnostic and interventional devices. The acWire may also function as an alignment tool by providing a reference plane of anatomical structure of interest (i.e. the aortic
valve). MediValve previously announced clearance of a CE pre-market application for the acWire.
“I am impressed with the acWire technology and the promise it holds for positioning diagnostic and interventional devices during heart catheterization procedures,” Carlos Ruiz, M.D., Ph.D., professor and chief of structural and congenital heart disease, North Shore-LIJ Hofstra University, North Shore Health System and Lenox Hill Hospital, New York City. “I look forward to evaluating the acWire in our clinic."
The MediValve acWire Guidewire is a single-use, fully disposable medical device utilizing shape-memory alloy technology intended to enable identification of cardiovascular structures utilizing existing imaging methods. Use of acWire employs methods and techniques currently utilized by interventional cardiologists to access the cardiovascular system. Once directed to a selected cardiovascular location, acWire is deformable under fluoroscopy to identify a desired specific anatomical landmark for subsequent therapeutic treatment by the clinician. AcWire is designed to facilitate the accurate placement and alignment of medical devices in the cardiovascular system during diagnostic and interventional procedures.
acWire received CE clearance November 2013.
For more information: www.medivalve.com