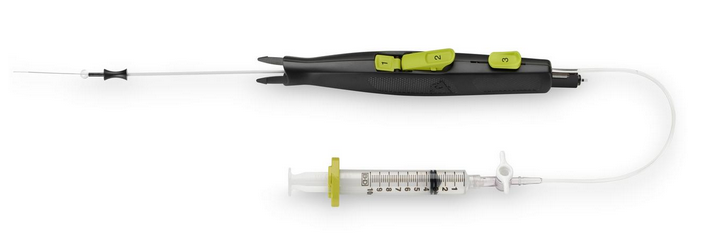
March 21, 2014 — AccessClosure plans to launch the Mynx Ace Vascular Closure Device at the 2014 American College of Cardiology meeting, March 29-31. It is an upgrade of the company’s Mynx mechanical vascular closure product. It is designed to provide patient comfort and uses an easy-to-use deployment system to seal femoral artery access sites. The device helps reduce time to hemostasis and ambulation in patients who have undergone diagnostic or interventional endovascular procedures.
The Mynx Ace uses an extravascular sealant plug for closure. The vendor’s Grip Technology actively adheres to the artery for secure mechanical closure and is completely extravascular. It is made of a bioresorbable material that dissolves within 30 days.
For information: www.accessclosure.com


 November 14, 2025
November 14, 2025 









