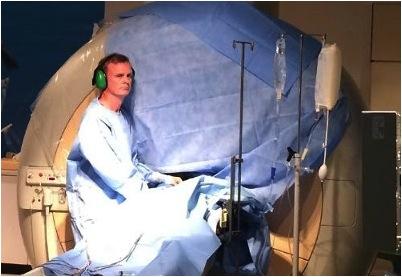
October 15, 2014 — Imricor Medical Systems, Inc. announced the first three cardiac ablation procedures were completed in the first clinical study that is evaluating the feasibility of their magnetic resonance (MR)-enabled products to treat atrial flutter. Prof. Reza Razavi, head of the Division of Imaging Sciences and Biomedical Engineering, King's College London, is the principal investigator for the study and performed the procedures along with Mark O'Neill, professor of cardiac electrophysiology and consultant cardiologist, Guy's and St. Thomas' NHS Trust in the United Kingdom, The prospective pilot study will enroll up to 15 patients at this center.
The Vision-MR Ablation Catheter and Advantage-MR EP Recorder/Stimulator System are currently being evaluated for the treatment of atrial flutter. The Vision-MR Ablation Catheter looks, feels and functions like a conventional ablation catheter. The Advantage-MR EP Recording/Stimulator System is also MR-enabled to avoid dangerous electromagnetic interactions with the MRI scanner and provide clear intra-cardiac electrograms and interference-free MR images. The system delivers the needed MR-enabled recording and pacing functions. Both products are used in conjunction with the iSuite image guidance platform that is provided by Philips.
"There have been many technical advances that have gone into this technology in order to create a system suitable for clinical use, and it has performed extremely well in the early phases of clinical trials. This is a groundbreaking study and we believe that there is a great future for MR-guided heart rhythm interventions. Not only is the requirement for X-rays removed, but for the first time the clinician is able to see the detailed anatomy of the heart, in real-time, during the procedure, and the therapeutic ablation lesions that have been created. The impact that such information could have on complex ablation procedures is enormous. We hope that this is a great step forward in the care and treatment of patients with abnormal heart rhythms," said Razavi.
For more information: www.imricor.com



 January 05, 2026
January 05, 2026 









