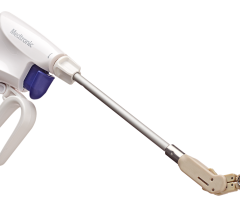
March 25, 2015 — Mount Sinai experts were the first in the eastern United States on March 23 to implant the Watchman device, a newly U.S. Food and Drug Administration (FDA)-approved device that prevents stroke in patients with arrhythmia.
The new device is designed to address atrial fibrillation, a malfunction that throws the heart out of rhythm, causing the upper chambers to beat too quickly. Often seen in aging patients, it interferes with electrical signals that drive the heartbeat. Patients with the condition have a five-times greater risk of stroke, and a greater chance of having a more serious stroke.
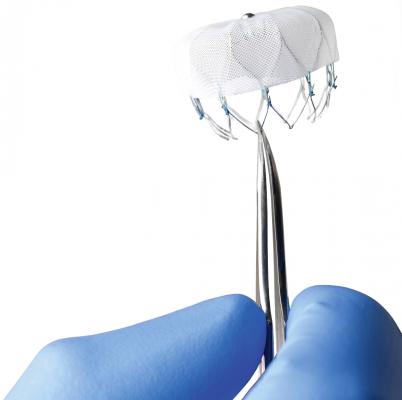
The Watchman device, the size of a quarter and shaped like a parachute, is implanted into the heart to close off the left atrial appendage, a blind pouch of heart tissue where blood clots form and can then break off and travel in the blood stream to the brain and cause strokes. The device is inserted into the heart through a vein in the leg during a one-time, minimally invasive, catheter-based procedure in the electrophysiology laboratory.
“Blood-thinning medications like warfarin or Coumadin are quite effective in reducing the risk for stroke in patients with atrial fibrillation. However, many of our patients cannot, or will not, tolerate these medications because of the incidence of bleeding. The Watchman device is a solution for such atrial fibrillation patients, since it similarly reduces their stroke risk, but averts their need for a lifetime of blood-thinners,” said Vivek Reddy, M.D., director of arrhythmia services at The Mount Sinai Hospital and the Mount Sinai Health System. Reddy was the electrophysiologist who led the procedure.
Reddy served as co-principal investigator for national clinical trials testing the Watchman device. The recently published results of these trials revealed that the device offers an efficient alternative to warfarin, and prevents strokes just as well. Patients with the device implanted also had a 60 percent reduction in cardiovascular mortality and 34 percent reduction in all-cause mortality.
Warfarin is the most common treatment currently used to reduce stroke risk in patients with atrial fibrillation. However, warfarin is not well-tolerated by all patients and has significant risk for bleeding complications, and patients need to be closely monitored with bi-weekly blood tests.
“This advanced technology will be truly life-changing for many atrial fibrillation patients, freeing them from not only the dangers of stroke risk, but also the daily challenges of long-term blood-thinning medication therapy,” says Valentin Fuster, M.D., Ph.D., director of Mount Sinai Heart and physician-in-chief of The Mount Sinai Hospital.
For more information: www.mountsinai.org


 April 04, 2024
April 04, 2024