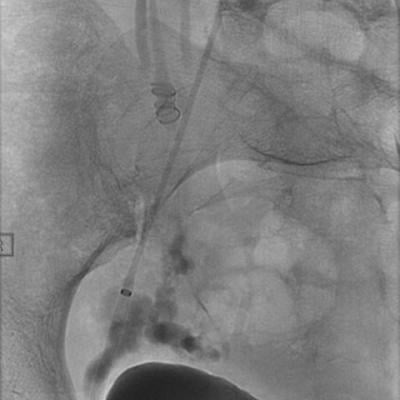
Image courtesy of ArtVentive Medical Group Inc.
February 24, 2016 — ArtVentive Medical Group Inc. announced enrollment in the ongoing ArtVentive EOS Endoluminal Occlusion System, OCCLUDE post-market surveillance study.
The study is dedicated to further advancement of use of the ArtVentive EOS embolization device in treating venous and arterial cases where the precise placement and immediate, efficient vessel embolization are paramount.
The first study case was performed at The University Hospital, Leuven, Belgium, where Geert Maleux, M.D., successfully treated a patient with the ArtVentive EOS device. Maleux, professor in the Department of Radiology stated, "The ArtVentive EOS device enhances our ability to treat challenging embolization cases. It offers immediate and complete occlusion. The operator can have confidence that the target vessel is occluded. The ArtVentive EOS technology allows for shorter procedure times and potentially reduced radiation exposure for both the physician and patient."
The device is catheter-based, self-expandable, and facilitates permanent or temporary occlusion of peripheral body lumens, cavities occurring within the body's vascular system and organ network.
For more information: www.artventivemedical.com


 November 14, 2025
November 14, 2025 









