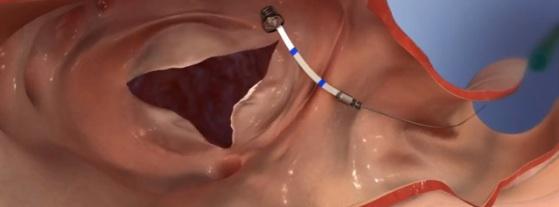
September 22, 2016 — 4Tech Inc. announced that its TriCinch device has been used in the world’s first-ever successful transcatheter tricuspid valve repair without the use of transesophageal echocardiography (TEE) or general anesthesia to successfully treat tricuspid regurgitation (TR). The TriCinch implant took less than one hour and allowed substantial reduction of TR.
The ongoing feasibility study is being conducted at San Raffaele Hospital and in other sites in Italy and in Europe. Investigators for the study include Azeem Latib, M.D., senior interventional cardiologist; Eustachio Agricola, M.D., senior echocardiographist; and Paolo Denti, M.D., hybrid cardiovascular surgeon.
“Our patient was at very high risk for surgical treatment, and she was referred to us by a cardiac surgeon,” said Agricola. “Equally critical, she was unable to undergo TEE because of an esophageal diverticulum and thus there were extremely limited options for offering her any treatment.”
“Fortunately, the extensive experience of the San Raffaele team with transcatheter treatment of the tricuspid valve and cardiac imaging allowed for the treatment,” added Latib. “The successful treatment of this case opens up new options for minimally invasive treatment of patients with FTR (functional tricuspid regurgitation).”
TR is a difficult-to-manage, age-related disease in which blood “backflows” into the right side of the heart. Today’s standard of care for TR is medical management. Surgical intervention is very high-risk. In-hospital death post-cardiac surgery for isolated TR can be as high as 37 percent. Unfortunately, TR patients tend to be non-compliant with their medications. Thus, TR and related complications induce substantial healthcare spending due to frequent re-hospitalizations. Furthermore, TR leads to chronic renal failure and end-stage dialysis. The combination of these negative outcomes results in a significant unmet need for an interventional cardiology solution to TR.
The 4Tech TriCinch System for Transcatheter Tricuspid Valve Repair is in the early phase of development. It will not be available in the United States for clinical trials until further notice and is not available for sale.
For more information: www.4techtricuspid.com


 November 14, 2025
November 14, 2025 









