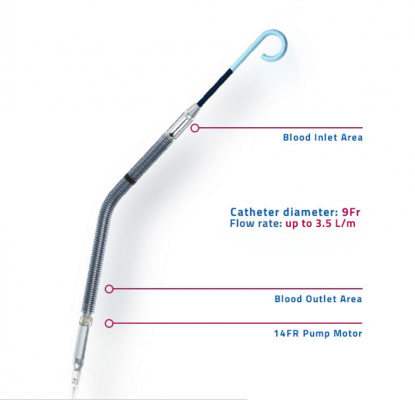
December 7, 2016 — Abiomed Inc. announced it has expanded its U.S. Food and Drug Administration (FDA) pre-market approval (PMA) for Impella heart pump use in high-risk percutaneous coronary interventions (PCI) to include the Impella CP heart pump. The Impella heart pumps provide the only minimally invasive treatment option with the unique ability to stabilize the patient's hemodynamics and unload the left ventricle of the heart, which allows the muscle to rest and recover its native function. The company said heart recovery is the ideal option for a patient's quality of life and has the ability to save costs for the healthcare system.[1,2,3]
In 2016, Impella CP's first FDA PMA approval was for up to four days of use to treat patients suffering from cardiogenic shock and is identical to Impella 2.5 (four days) and Impella 5.0 (six days) indications: The Impella 2.5, Impella CP, Impella 5.0 and Impella LD catheters, in conjunction with the Automated Impella Controller, are temporary ventricular support devices intended for short-term use (<4 days for the Impella 2.5 and Impella CP, and <6 days for the Impella 5.0 and Impella LD) and indicated for the treatment of ongoing cardiogenic shock that occurs immediately (<48 hours) following acute myocardial infarction (AMI) or open heart surgery as a result of isolated left ventricular failure that is not responsive to optimal medical management and conventional treatment measures, including volume loading and the use of pressors and inotropes, with or without IABP an intra-aortic balloon pump. The intent of the Impella system therapy is to reduce ventricular work and to provide the circulatory support necessary to allow heart recovery and early assessment of residual myocardial function.
Today, Abiomed announces the second Impella CP indication for FDA approval for high-risk PCI, identical to Impella 2.5: The Impella 2.5 and Impella CP are temporary (≤ 6 hours) ventricular support systems indicated for use during high risk percutaneous coronary interventions (PCI) performed in elective or urgent hemodynamically stable patients with severe coronary artery disease and depressed left ventricular ejection fraction, when a heart team, including a cardiac surgeon, has determined high risk PCI is the appropriate therapeutic option. Use of the Impella 2.5 and Impella CP in these patients may prevent hemodynamic instability which can result from repeat episodes of reversible myocardial ischemia that occur during planned temporary coronary occlusions and may reduce peri- and post-procedural adverse events.
In the U.S. alone, Abiomed estimates there is a large unmet need of approximately 121,000 high-risk patients annually who are chronically ill with advanced, inoperable heart disease such as severe coronary artery disease. Consensus publications and clinical guidelines from the American College of Cardiology and Society for Cardiac Angiography and Interventions have documented Impella heart pumps as the new standard of care in algorithms to treat High-Risk PCI. Impella 2.5 and Impella CP heart pumps are the only hemodynamic support devices proven safe and effective by the FDA for high-risk PCI and AMI cardiogenic shock.
"This latest approval for Impella expands the hemodynamic options for the cardiovascular community to effectively revascularize severely ill patients who have limited options and high mortality risk," said Jeffrey W. Moses, M.D., professor of medicine, Columbia University Medical Center. "Backed by clinical data and real world experience since 2008, interventional cardiologists working with their heart teams to identify complex PCI candidates can perform complete revascularization on previously untreatable patients to improve their quality of life and their native heart function."
Data submitted from an FDA IDE approved, randomized multicenter trial (Protect II) demonstrated that Protected PCI with Impella heart pumps reduced major adverse events (MACCE) by 29 percent[4], increased patient quality of life by 58 percent[5], and showed superior hemodynamics and improved cardiac power over the control arm (IABP). Additionally, multiple independent analyses reveal Impella usage is cost effective (ICER) or dominant (lowers absolute costs) in emergency patients. Benefits from Impella-supported procedures may also include a reduction in symptoms and class of heart failure, reduction of days in the hospital, and a reduction in readmissions due to fewer repeat procedures.
Data Supporting FDA Approval
In addition to the robust data submitted for the Impella 2.5 approval, including the FDA safety study PROTECT I and the Randomized Controlled Trial PROTECT II, the results from a retrospective data review of 72 patients supported with Impella CP and 637 patients treated with Impella 2.5 were submitted. Post-market surveillance will be conducted through the cVAD Registry. Additional clinical data submitted to the FDA by Abiomed for approval consideration came from the cVAD Registry, previously known as U.S. Impella registry, which contains nearly 3,000 patient records. The data collection from the registry includes Institutional Review Board (IRB) approval, complete data monitoring and Clinical Events Committee adjudication.
In addition to data submitted to the FDA, the Abiomed Impella Quality Assurance Program (IQ) includes a collection of observational data on over 95 percent of Impella patients since the heart pump's introduction to the United States in 2008. Today, this holds more than 45,000 Impella case entries. These FDA studies, IRB controlled registry, and Abiomed's quality database are helping to identify best practices and protocols that appear linked to the highest survival and native heart recovery rates at the hospitals with Impella.
For more information: www.abiomed.com
References:
1. Maini B, Gregory D, Scotti DJ, Buyantseva L. Catheter Cardiovasc Interv. 2014 May 1;83(6):E183-92.
2. Cheung A, Danter M, Gregory D. J Am Coll Cardiol. 2012;60(17_S):. doi:10.1016/j.jacc.2012.08.413.
3. Gregory D, Scotti DJ, de Lissovoy G, Palacios I, Dixon, Maini B, O'Neill W. Am Health Drug Benefits. 2013 Mar;6 (2):88-99.
4. Dangas GD, Kini AS, Sharma SK, et al. Am J Cardiol. 2014;113(2):222-228.
5. O'Neill WW, Kleiman NS, Moses J, et al. Circulation. 2012 Oct 2;126(14):1717-27.


 November 14, 2025
November 14, 2025 









